��Ŀ����
2010�꣬����ѡ��һ����ѧ��ʦ���½�����Ϊ��һ���֧�̹�����������ǿ������ѧУ֮��Ľ�����Ҳ�ٽ����½����ؽ�����ҵ�ķ�չ��������ͼ�ס�ͼ��������и��⣮
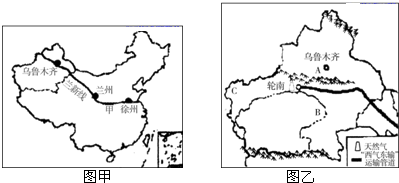
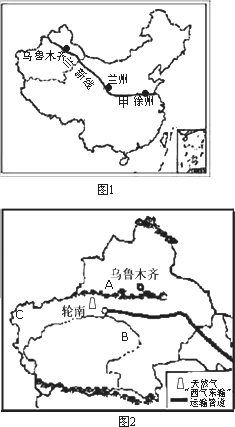
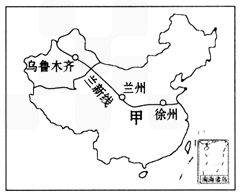
��1���½�λ���ҹ��Ĵ���������е� ������
��2����ʦ�������ݳ�����ǰ���½�����ô��������·����Ӧ������ �ߺ������ߵȣ�
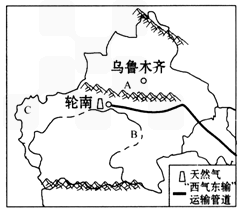
��3���г��н������н���Խ�ĵ����ֽ��߰��� ������ĸ����
�ٺں�-�ڳ���
���ȴ������ȴ��ķֽ���
�ۼ�������Ǽ������ķֽ���
�����������������ķֽ���
A���٢ڢ�B���ڢۢ�C���٢ۢ�D���٢ڢ�
��4���½���������ɽ����ͼ����A��ʾ�� ɽ����B�� �ӣ��ҹ����������������½���C ��ԭ�ϣ�
��5������ͬѧ��˵��������ȷ����
A����������ʦ�����ﶼҪ���ϣ���Ϊ�½����´���½�����������սϲ����ϲ��
B��ǿǿ��Ϊ�½��ij��ж������ֲ�
C������˵���½���Ҫ������������ά����壮
D���������ã��½����ƶ�����Զ��������ľ��÷�չû�а���
��6������д�������½�����ɫ�Ϲ� �� ��

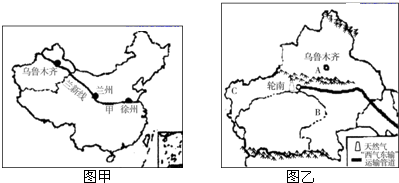
��1���½�λ���ҹ��Ĵ���������е�
��2����ʦ�������ݳ�����ǰ���½�����ô��������·����Ӧ������
��3���г��н������н���Խ�ĵ����ֽ��߰���
�ٺں�-�ڳ���
���ȴ������ȴ��ķֽ���
�ۼ�������Ǽ������ķֽ���
�����������������ķֽ���
A���٢ڢ�B���ڢۢ�C���٢ۢ�D���٢ڢ�
��4���½���������ɽ����ͼ����A��ʾ��
��5������ͬѧ��˵��������ȷ����
A����������ʦ�����ﶼҪ���ϣ���Ϊ�½����´���½�����������սϲ����ϲ��
B��ǿǿ��Ϊ�½��ij��ж������ֲ�
C������˵���½���Ҫ������������ά����壮
D���������ã��½����ƶ�����Զ��������ľ��÷�չû�а���
��6������д�������½�����ɫ�Ϲ�
�������½������ŷ��½�����µ���仯���ձ仯���ܴ�ȫ������죬����ǿ�ң����ڽ�ˮϡ�٣�������ʢ��ũҵ��������������ȣ�����ľ��غ�������ر�Ե�й��ˮԴ�ĵش����ֲ����ڶ�����ޣ��������½�����Ҫũҵ����
����⣺��1���½��ش��ҹ�������λ���ҹ��Ĵ���������е�����������
��2�������ݵ��½���³ľ�����·������¤��-�����ߣ�
��3��¤��-�����ߴ�Խ���ҹ��˿ڵ������ߺں�-�ڳ��ߡ���������Ǽ������ķֽ��ߡ����������������ķֽ��ߣ���ѡ��C�������⣮��ѡC��
��4���½��ĵ�������Ϊ��ɽ�����裬������ɽ����AΪ��ɽ������ľ��������ҹ����������B����ľ�ӣ��ҹ����������������½���C������ԭ�ϣ�
��5���½������Դ�ḻ���ر���������Դ�̲���������ƶ������ѡ��D�������⣮��ѡD��
��6���½��������ϵ�ũ������Ҫ��С�����ס�������˺Ϲ������ܹϡ���³�����Ѹ�������ȫ����
�ʴ�Ϊ����1����������2��¤������3��C����4����ɽ������ľ����������5��D����6����³�����ѣ����ܹϣ�
��2�������ݵ��½���³ľ�����·������¤��-�����ߣ�
��3��¤��-�����ߴ�Խ���ҹ��˿ڵ������ߺں�-�ڳ��ߡ���������Ǽ������ķֽ��ߡ����������������ķֽ��ߣ���ѡ��C�������⣮��ѡC��
��4���½��ĵ�������Ϊ��ɽ�����裬������ɽ����AΪ��ɽ������ľ��������ҹ����������B����ľ�ӣ��ҹ����������������½���C������ԭ�ϣ�
��5���½������Դ�ḻ���ر���������Դ�̲���������ƶ������ѡ��D�������⣮��ѡD��
��6���½��������ϵ�ũ������Ҫ��С�����ס�������˺Ϲ������ܹϡ���³�����Ѹ�������ȫ����
�ʴ�Ϊ����1����������2��¤������3��C����4����ɽ������ľ����������5��D����6����³�����ѣ����ܹϣ�
������������Ҫ�����½��ĸſ�����һ���½���ˮԴ�������

��ϰ��ϵ�д�
 ABC����ȫ�ž�ϵ�д�
ABC����ȫ�ž�ϵ�д�
�����Ŀ
| |||||||||||||||||||||||||||||||||||||||||||||||
| 2010�꣬����ѡ��һ����ѧ��ʦ���½�����Ϊ��һ���֧�̹�����������ǿ������ѧУ֮��Ľ�����Ҳ�ٽ����½����ؽ�����ҵ�ķ�չ����ͼ1��ͼ2������и��⡣ | ||
| ||
| ��1���½�λ���ҹ��Ĵ���������е�___________������ | ||
| ��2����ʦ�������ݳ�����ǰ���½�����ô��������·����Ӧ������____________�ߺ������ߵȡ� | ||
| ��3���г��н������н���Խ�ĵ����ֽ��߰��� �ٺںӡ��ڳ��ߢ��ȴ������ȴ��ķֽ��ߢۼ�������Ǽ������ķֽ��ߢ����������������ķֽ��� | ||
|
[ ] | ||
| A���٢ڢ� B���ڢۢ� C���٢ۢ� D���٢ڢ� | ||
| ��4���½���������ɽ����ͼ2��A��ʾ��______________ɽ����B��_______________�ӣ��ҹ����������������½���C___________________��ԭ�ϡ� | ||
| ��5������ͬѧ�����½���˵��������ȷ���� | ||
|
[ ] | ||
| A����������ʦ�����ﶼҪ���ϣ���Ϊ�½���Ҫ���´���½�����������սϲ����ϲ�� B��ǿǿ��Ϊ�½��ij��ж������ֲ� C������˵���½���Ҫ������������ά����� D���������ã�������ƶ�����Զ��������ľ��÷�չû�а��� | ||
| ��6������д�������½�����ɫ�Ϲ�______________��________________�� |