��Ŀ����
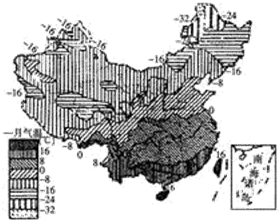 �����ҹ�һ�·�ƽ�����·ֲ�ͼ�����ش����и��⣮
�����ҹ�һ�·�ƽ�����·ֲ�ͼ�����ش����и��⣮��1����ͼ���ҹ������ߵķֲ����Կ�����0������ߴ�����
��2��һ�·��ҹ�������͵ĵط���
A����ظ�ԭ B��Į�� C�����ҵ� D���½�����
��3��һ�·ݣ��Ĵ���ص�����һ��Ҫ�ȳ���������ƽԭ�߳���Լ4��C���ң�����Ҫԭ����
A�����ܵ�̫������� B�����Ƹ�
C������ɽ���赲���ܶ�����Ӱ����� D��γ�ȵ�
��4���ҹ�7��ƽ��������͵�������
��5�������¶ȴ�����Ҫָ����
��������γ��λ�úͶ������Ӱ�죬�������ҹ�Խ���������¾�Խ�ͣ������ϱ����²�����ļ�̫��ֱ�䱱������������̫���߶Ȳ������Ϸ��Ͷ��٣����������糤ȴ���Ϸ�������˱����õ��Ĺ��Ȳ������Ϸ��٣������ļ�����ظ�ԭ�⣬�ҹ��ϱ��ձ���£��ϱ��²�����ں��θߣ���ظ�ԭ��Ϊ�ļ�������͵ĵ�����
����⣺��ͼ��֪��
��1���ҹ�0������ߴ���������-�����߷ֲ��������Ա�ƽ�������� 0�����£�Խ��������Խ�ͣ���������ƽ�������� 0�����ϣ�Խ��������Խ�ߣ��ϱ����²�� 50�棬�ü����ҹ����µķֲ��ص����ϱ����²����
��2���ҹ��������·ֲ��ص����ϱ��������ܴ�Խ��������Խ�ͣ����һ�·��ҹ�������͵ĵط���Į�ӣ���ѡ��B�������⣮
��3�������Ĵ���ر���ɽ���赲���ܶ�����Ӱ����������һ�·ݣ��Ĵ���ص�����һ��Ҫ�ȳ���������ƽԭ�߳���Լ4��C���ң���ѡ��C�������⣮
��4�����θ����µͣ��ҹ�7��ƽ��������͵���������ظ�ԭ���ҹ��ļ����µķֲ��ص����ϱ��²�С��ȫ���ֵ����ձ���£�
��5�������¶ȴ�����Ҫָ���ǻ��£����ȴ���ů�´��ķֽ������й�1��0������ߴ���һ�£�������ĵ����������¶ȴ���ů�´����������ڵػش𣩣�
�ʴ�Ϊ����1������-���ӣ�0���ͣ��ϱ����²����2��B����3��C����4����ظ�ԭ���²�С�����£���5�����£�0��ů�´���
��1���ҹ�0������ߴ���������-�����߷ֲ��������Ա�ƽ�������� 0�����£�Խ��������Խ�ͣ���������ƽ�������� 0�����ϣ�Խ��������Խ�ߣ��ϱ����²�� 50�棬�ü����ҹ����µķֲ��ص����ϱ����²����
��2���ҹ��������·ֲ��ص����ϱ��������ܴ�Խ��������Խ�ͣ����һ�·��ҹ�������͵ĵط���Į�ӣ���ѡ��B�������⣮
��3�������Ĵ���ر���ɽ���赲���ܶ�����Ӱ����������һ�·ݣ��Ĵ���ص�����һ��Ҫ�ȳ���������ƽԭ�߳���Լ4��C���ң���ѡ��C�������⣮
��4�����θ����µͣ��ҹ�7��ƽ��������͵���������ظ�ԭ���ҹ��ļ����µķֲ��ص����ϱ��²�С��ȫ���ֵ����ձ���£�
��5�������¶ȴ�����Ҫָ���ǻ��£����ȴ���ů�´��ķֽ������й�1��0������ߴ���һ�£�������ĵ����������¶ȴ���ů�´����������ڵػش𣩣�
�ʴ�Ϊ����1������-���ӣ�0���ͣ��ϱ����²����2��B����3��C����4����ظ�ԭ���²�С�����£���5�����£�0��ů�´���
������������Ҫ�����ҹ��������µķֲ��ص㣬ͬʱ������ѧ���Ķ�ͼ������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 ���й���������ͼ���ش�
���й���������ͼ���ش� ���й���������ͼ���ش�
���й���������ͼ���ش�
