��Ŀ����
��ŷ���������ձ�����ͼ��ͼ1��ͼ2�����ij�����������ͼ��ͼ3����������и��⣮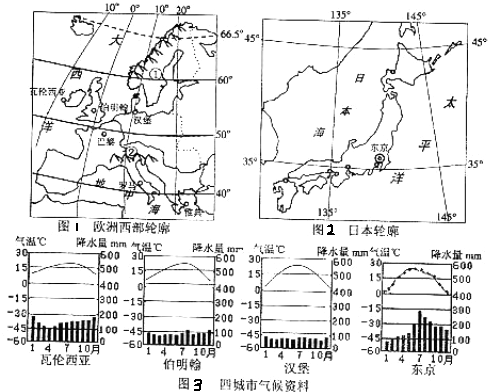
��1��ͼ�����ִ����ĵ����������ƣ�����
��2���������ǡ������������������й�ͬ������������
�������������������Ӱ��������
��3���������붫�����Ǻ������У�Ϊʲô�������½�ˮ�����ȣ�
��4���ձ����ɽ�����ԭ��
������ŷ������������ͱ�������ů����Ӱ�죬ȫ���ºͶ��꣬Ϊ���͵��´�����������ŷ�������Ĵ������ذ������ž��뺣��Խ��ԽԶ�����������ɺ��������½��ת����ŷ���������ϱ�������ɽ�طֲ��⣬�����в���������ƽԭΪ�������ֵ��ζ������Ӱ�������ں���ʪ�����������½�ڲ���ŷ���������ڳ��������缰ů��Ӱ�죬�����ºͶ��꣬���ղ��㣬��ˮ�Ϸḻ���ʺ�����ҵ�ķ�չ��
����⣺��1����ͼ����Ϊ˹������ά�ǰ뵺����Ϊ������˹ɽ����
��2����ͼ��֪���������ǡ������������������й�ͬ����������������ϲ�С����ˮ���ȣ�ȫ����ůʪ����������λ�ü��������Ϸֲ���֪��ŷ����������������������ϲ�������ˮ�����٣���Ҫ�����ں�½������ɣ�
��3������λ�����ȴ���������������ˮ�༯�����ļ���
��4���ձ���ɽ������ԭ����λ����ŷ�����̫ƽ����Ľ��紦���ؿǻ�Ծ��
�ʴ�Ϊ����1��˹������ά�ǣ�������˹��
��2��ȫ����ůʪ��������ϲ�������ˮ�����٣��ຣԶ����ͬ��
��3������λ�����ȴ���������������ˮ�༯�����ļ���
��4��������ŷ����̫ƽ���齻��ش���
��2����ͼ��֪���������ǡ������������������й�ͬ����������������ϲ�С����ˮ���ȣ�ȫ����ůʪ����������λ�ü��������Ϸֲ���֪��ŷ����������������������ϲ�������ˮ�����٣���Ҫ�����ں�½������ɣ�
��3������λ�����ȴ���������������ˮ�༯�����ļ���
��4���ձ���ɽ������ԭ����λ����ŷ�����̫ƽ����Ľ��紦���ؿǻ�Ծ��
�ʴ�Ϊ����1��˹������ά�ǣ�������˹��
��2��ȫ����ůʪ��������ϲ�������ˮ�����٣��ຣԶ����ͬ��
��3������λ�����ȴ���������������ˮ�༯�����ļ���
��4��������ŷ����̫ƽ���齻��ش���
���������⿼��ŷ�����������������εȣ���ͼ������ϼ�

��ϰ��ϵ�д�
�����Ŀ


