摘要:886
网址:http://m.1010jiajiao.com/timu_id_127362[举报]
已知:
O2(g) = O2+(g)+e- △H1= +1175.7 kJ/mol
PtF6-(g) =PtF6(g)+e- △H2= +771.1 kJ/mol
O2+(g)+PtF6-(g) =O2PtF6(s) △H3= -482.2 kJ/mol
则反应O2(g)+PtF6(g) = O2PtF6(s)的△H是
O2(g) = O2+(g)+e- △H1= +1175.7 kJ/mol
PtF6-(g) =PtF6(g)+e- △H2= +771.1 kJ/mol
O2+(g)+PtF6-(g) =O2PtF6(s) △H3= -482.2 kJ/mol
则反应O2(g)+PtF6(g) = O2PtF6(s)的△H是
[ ]
A.77.6 kJ
B.-77.6 kJ/mol
C.+77.6kJ/mol
D.-886.8kJ/mol
查看习题详情和答案>>
B.-77.6 kJ/mol
C.+77.6kJ/mol
D.-886.8kJ/mol
已知:O2(g) = O2+(g)+e- △H1= +1175.7 kJ·mol-1
PtF6-(g) =PtF6(g)+e- △H2= +771.1 kJ·mol-1
O2+(g)+PtF6-(g) =O2PtF6(s) △H3= -482.2 kJ·mol-1
则反应O2(g)+PtF6(g) = O2PtF6(s)的△H是
PtF6-(g) =PtF6(g)+e- △H2= +771.1 kJ·mol-1
O2+(g)+PtF6-(g) =O2PtF6(s) △H3= -482.2 kJ·mol-1
则反应O2(g)+PtF6(g) = O2PtF6(s)的△H是
[ ]
A.77.6 kJ
B.-77.6 kJ·mol-1
C.+77.6kJ·mol-1
D.-886.8kJ·mol-1
查看习题详情和答案>>
B.-77.6 kJ·mol-1
C.+77.6kJ·mol-1
D.-886.8kJ·mol-1
已知:O2(g) = O2+(g)+e-  H1=" +1175.7" kJ·mol-1
H1=" +1175.7" kJ·mol-1
PtF6-(g) =PtF6(g)+e-  H2=" +771.1" kJ·mol-1
H2=" +771.1" kJ·mol-1
O2+(g)+PtF6-(g) =O2PtF6(S)  H3=" -482.2" kJ·mol-1
H3=" -482.2" kJ·mol-1
则反应O2(g)+PtF6(g) = O2PtF6 (s)的 H是
H是
| A.77.6 kJ | B.-77.6 kJ·mol-1 | C.+77.6kJ·mol-1 | D.-886.8kJ·mol-1 |
已知:O2(g) = O2+(g)+e- 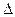 H1=" +1175.7" kJ·mol-1
H1=" +1175.7" kJ·mol-1
PtF6-(g) =PtF6(g)+e-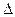 H2=" +771.1" kJ·mol-1
H2=" +771.1" kJ·mol-1
O2+(g)+PtF6-(g) =O2PtF6(S)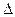 H3=" -482.2" kJ·mol-1
H3=" -482.2" kJ·mol-1
则反应O2(g)+PtF6(g) = O2PtF6 (s)的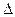 H是
H是
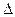 H1=" +1175.7" kJ·mol-1
H1=" +1175.7" kJ·mol-1PtF6-(g) =PtF6(g)+e-
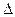 H2=" +771.1" kJ·mol-1
H2=" +771.1" kJ·mol-1O2+(g)+PtF6-(g) =O2PtF6(S)
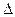 H3=" -482.2" kJ·mol-1
H3=" -482.2" kJ·mol-1则反应O2(g)+PtF6(g) = O2PtF6 (s)的
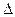 H是
H是| A.77.6 kJ | B.-77.6 kJ·mol-1 | C.+77.6kJ·mol-1 | D.-886.8kJ·mol-1 |
已知:O2(g) = O2+(g)+e-  H1=" +1175.7" kJ·mol-1
H1=" +1175.7" kJ·mol-1
PtF6-(g) =PtF6(g)+e-  H2=" +771.1" kJ·mol-1
H2=" +771.1" kJ·mol-1
O2+(g)+PtF6-(g) =O2PtF6(S)  H3=" -482.2" kJ·mol-1
H3=" -482.2" kJ·mol-1
则反应O2(g)+PtF6(g) = O2PtF6 (s)的 H是( )
H是( )
| A.77.6 kJ | B.-77.6 kJ·mol-1 | C.+77.6kJ·mol-1 | D.-886.8kJ·mol-1 |