网址:http://m.1010jiajiao.com/timu3_id_415641[举报]
已知:Fe2O3(s)+C(s)===CO2(g)+2Fe(s) ΔH=+234.14 kJ·mol-1
C(s)+O2(g)===CO2(g) ΔH=-393.5 kJ·mol-1
则2Fe(s)+O2(g)===Fe2O3(s)的ΔH是
A.-824.39 kJ·mol-1 B.+ 627.6 kJ·mol-1
C。-744.7 kJ·mol-1 D.-169.4 kJ·mol-1
查看习题详情和答案>>
已知:Fe2O3(s)+C(s)===CO2(g)+2Fe(s) ΔH=+234.14 kJ·mol-1
C(s)+O2(g)===CO2(g) ΔH=-393.5kJ·mol-1
则2Fe(s)+O2(g)===Fe2O3(s)的ΔH是
A.-824.39 kJ·mol-1 B.+ 627.6kJ·mol-1
C。-744.7 kJ·mol-1 D.-169.4kJ·mol-1
查看习题详情和答案>>
已知:Fe2O3(s)+![]() C(s) =
C(s) = ![]() CO2(g)+2Fe(s) H =+234.1 kJ·mol-1
CO2(g)+2Fe(s) H =+234.1 kJ·mol-1
 C(s)+O2(g) = CO2(g) H =-393.5 kJ·mol-1
C(s)+O2(g) = CO2(g) H =-393.5 kJ·mol-1
则2Fe(s)+![]() O2(g) = Fe2O3(s)的H是 ( )
O2(g) = Fe2O3(s)的H是 ( )
A.-824.4 kJ·mol-1 B.-627.6 kJ·mol-1
C.-744.7 kJ·mol-1 D.-169.4 kJ·mol
查看习题详情和答案>>
已知:Fe2O3(s)+C(s)===CO2(g)+2Fe(s) ΔH=+234.14 kJ·mol-1
C(s)+O2(g)===CO2(g) ΔH=-393.5 kJ·mol-1
则2Fe(s)+O2(g)===Fe2O3(s)的ΔH是
| A.-824.39 kJ·mol-1 | B.+ 627.6 kJ·mol-1 |
| C.-744.7 kJ·mol-1 | D.-169.4 kJ·mol-1 |
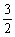 C(s) =
C(s) =  C(s)+O2(g) = CO2(g)
H =-393.5 kJ·mol-1
C(s)+O2(g) = CO2(g)
H =-393.5 kJ·mol-1