摘要:10.已知H2.ΔH====-184.6KJ·mol-1 则反应HCl(g)===的ΔH是 A.+184.6 kJ·mol-1 B.-369.2 kJ·mol-1 C.+92.3 kJ·mol-1 D.-92.3 kJ·mol-1
网址:http://m.1010jiajiao.com/timu3_id_385270[举报]
已知H2(g)+Cl2(g)=2HCl(g),△H=-184.6kJ·mol-1,则反应HCl(g)=1/2H2(g)+1/2Cl2(g)的△H为……( )
A、+184.6kJ·mol-1 B、-92.3kJ·mol-1 C、-369.2kJ·mol-1 D、+92.3kJ·mol-1
查看习题详情和答案>>
已知H2(g)+C12(g)====2HCl(g);ΔH=-184.6kJ·mol-1,则反应HCl(g)====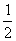 H2(g)+Cl2(g)的ΔH为( )
H2(g)+Cl2(g)的ΔH为( )
A.+184.6 kJ·mol-1 B.-92.3 kJ·mol-1
C.+92.3 kJ·mol D.-369.2 kJ·mol-1
查看习题详情和答案>>已知H2(g)+Cl2(g)=2HCl(g) △H=-184.6kJ·mol-1, 则反应HCl(g)=1/2H2(g)+1/2Cl2(g)的△H为
[ ]
A.+184.6kJ·mol-1
B.-92.3kJ·mol-1
C.+92.3kJ
D.+92.3kJ·mol-1
查看习题详情和答案>>
B.-92.3kJ·mol-1
C.+92.3kJ
D.+92.3kJ·mol-1