��Ŀ����
��ѧ�ҽ��˵��������ػ����������������ţ���ܾ��ѣ����ɹ���ţ������ȡ�������������أ�������������ͼ��ʾ�����ͼ�ش𡣣���֪����øI��ʶ�����к��е��ǣ�G��GATCC�� ������øII��ʶ�����к��е��ǣ���GATC������
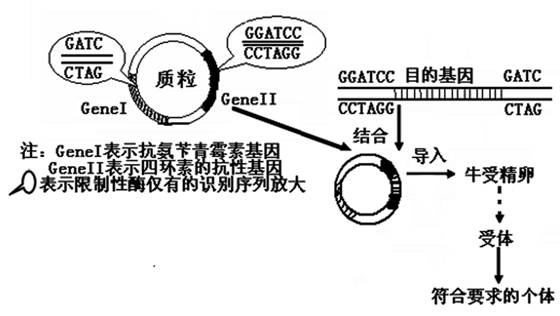
��1����ȡĿ�Ļ������Ҫ;����������������ϸ������ȡ�ͣߣߣߣߣߣߣߣߣߣߣߣߡ�
��2����ͼ�������ڹ������������Ĺ����У�Ŀ�Ļ����ãߣߣߣߣߣߣߣ߽������и
��3�����ܵľ��Ӻ���ϸ�����������ȷ����ߣߣߣߣߣ߷�Ӧ��֮���ӽӴ��ѻ�Ĥ������һ�����ѽ�ϣ��ڸù����У��ߣߣߣߣߣߣߣߣߣߣߣߺͣߣߣߣߣߣߣߣߣߣߣߣ߿��Է�ֹ�����������ѡ�
��4��ͼ�С����塱�ǣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�������Ҫ��ĸ��塱��ָ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��5����ţ�ܾ��ѽ����˹�������һ���ѡ��ߣߣߣߣߣߣߣߣߣߣߣ��ڵġ��������õ���̥������ֲ��
��1��������ת¼�ȷ������У��˹��ϳ�
��2������ø��
��3�����塡��������Ӧ�����ѻ�Ĥ�������
��4�������ĸţ���ôƼ��ش������ĸţ�������ܷ��ں�����������ţ�̵�ĸţ
��5��ɣ�������

��ϰ��ϵ�д�
�����Ŀ


 ��GATC
��GATC

