��Ŀ����
�±����������������һ������Һ�䷽��
| Ca(NO3)2 | MgSO4 | KH2PO4 | |||
| 10 g | 0.25 g | 0.12 g | |||
| KCl | FeCl3 | K2HPO4 | H2O | ||
| 0.12 g | 0.005 g | 0.12 g | 1 000 mL | ||
��1��Mg���Ͳ˱���Ĵ���Ԫ�أ�ȱMgʱ���Ͳ˽����ϳ�Ҷ���ء�Ϊ�˹۲�ȱMgʱ��ֲ���Ӱ�죬Ӧ����������Һ���䷽��θĶ���_____________________��
��2���ü�������״��������ͬ���Ͳˡ���ֻ�����ס����ܵȣ���������ʵ�鲽����֤��Mg���Ͳ˵ı���Ԫ�ء�
��һ����������ȫ����Һ��ȱMg����Һ���ֱ����A��B�����������С�
�ڶ�����_____________________��
Ԥ�ڽ����_____________________��
���ۣ�_____________________��
��1��ȥ��MgSO4������������K2SO4 ��2���ڶ�����ѡ������ͬ���Ͳ��������A��B�����У�����ͬ���������� Ԥ�ڽ����A�����Ͳ�����������B�����Ͳ���Ҷ��� ���ۣ�Mg���Ͳ˱���Ŀ���Ԫ��
����:
�����ϱ����Եó���Ҫ����ȱMg����Һ��Ӧ������Һ�е�MgSO4ȥ����ͬʱ����������K2SO4������ɵڶ���ʵ�����ʱ��Ӧ��ֿ��ǵ�һ����ԭ��ѡȡ������ͬ�Ͳ��������A��B�����У�����ͬ����������������Ԥ��A�����Ͳ�����������B�����Ͳ���Ҷ��ƣ�Mg�ǿ������õĿ���Ԫ�أ����Ӷ��ó����ۣ�Mg���Ͳ˱���Ŀ���Ԫ�ء�

�±����������������һ������Һ�䷽����ش��������⣺
| Ca��NO3��2����������������������������������������1��0 g MgSO4������������������������������������������0��25 g KH2PO4������������������������������������������0��25 g KCl����������������������������������������������0��12 g FeCl3��������������������������������������������0��005 g H2O����������������������������������������������1000 mL |
��1���ô�����Һ����ij�ߵ�ֲ��ʱ������Ҫ������Һ��ͨ���������һ��ʩ��Ŀ�������Դٽ�_____________�������ڶԿ���Ԫ�ص����ա�Ҫʹ��ֲ���������ã����ݹ�����õı����������������˵��¶��⣬����Ҫ_____________��_____________��
��2��ֲ������������Һ�п�������ʱ����Ҫ��ͨ��_____________��ʽ���еġ�����һ��ʱ�������Һ�������Ca2+�϶࣬��NO![]() ���٣���һ������ϸ��Ĥ��_____________�йء�
���٣���һ������ϸ��Ĥ��_____________�йء�
��3������ȥ������Һ�е�MgSO4����ֱ��Ӱ��ֲ������_____________�ĺϳɡ�
��4�����䷽������ֲ������Ĵ�������Ԫ����_____________����Ԫ����_____________��
�±����������������һ������Һ�䷽����ش��������⣺
| Ca(NO3)2������������������������������������������������������������1.0�� |
| MgSO4��������������������������������������������������������������0.25�� |
| KH2PO4��������������������������������������������������������������0.25�� |
| KCl����������������������������������������������������������������0.12�� |
| FeCl3��������������������������������������������������������������0.005�� |
| H2O����������������������������������������������������������������1000���� |
��1���ô�����Һ����ij�ߵ�ֲ��ʱ������Ҫ������Һ��ͨ���������һ��ʩ��Ŀ�������Դٽ� �������ڶԿ���Ԫ�ص����ա�Ҫʹ��ֲ���������ã����ݹ��������������������������˵��¶��⣬����Ҫ �� ��
��2��ֲ������������Һ�еĿ�������ʱ����Ҫ��ͨ�� ��ʽ���еġ�����һ��ʱ�������Һ�д���Ca2+�϶࣬��NO![]() ���٣���һ������ϸ��Ĥ�� �йء�
���٣���һ������ϸ��Ĥ�� �йء�
��3������ȥ������Һ�е�MgSO4,��ֱ��Ӱ��ֲ������ �ĺϳɡ�
��4�����䷽������ֲ������Ĵ�������Ԫ���� ����Ԫ���� ��
�±����������������һ������Һ�䷽����ش��������⣺
|
Ca��NO3��2����������1.0 g MgSO4��������������0.25 g KH2PO4��������������0.25 g KCl�������� ���� ����0.12 g FeCl3����������������0.005 g H2O����������������1000 mL |
��1���ô�����Һ����ij�ߵ�ֲ��ʱ������Ҫ������Һ��ͨ���������һ��ʩ��Ŀ�������Դٽ�_____________�������ڶԿ���Ԫ�ص����ա�Ҫʹ��ֲ���������ã����ݹ�����õı����������������˵��¶��⣬����Ҫ_____________��_____________��
��2��ֲ������������Һ�п�������ʱ����Ҫ��ͨ��_____________��ʽ���еġ�����һ��ʱ�������Һ�������Ca2+�϶࣬��NO ���٣���һ������ϸ��Ĥ��_____________�йء�
���٣���һ������ϸ��Ĥ��_____________�йء�
��3������ȥ������Һ�е�MgSO4����ֱ��Ӱ��ֲ������_____________�ĺϳɡ�
��4�����䷽������ֲ������Ĵ�������Ԫ����_____________����Ԫ����_____________��
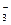 ���٣���һ������ϸ��Ĥ��______
���٣���һ������ϸ��Ĥ��______ _______�йء�
_______�йء�