��Ŀ����
��12�֣��������غ͡��ĵð���ҩ�ﶼ����Ӱ������Ļ��ij��ȤС��Χ���������غ��ĵð��Ĺ��ܽ�����ʵ���о���������±���ʾ��������ʵ�鱨�沢�ش�������⣺��+��ʾ��ע�䣬����ʾû��ע�䡣��
ע��ҩ������
��� �ĵð� �������� ������(��103mL)
A - - 2��O
B + - 1.7
C - + 2.5[
D + + 2.1
�� ʵ��Ŀ�ģ�̽��_______ ___����2�֣�
�� �����þߣ�С������ֻ��ע��������������װ��(������ͼ)������������Һ���ĵð���Һ(��������ˮ����)��

�� �������裺
��ѡȡ��С������״����ͬ��С��20ֻ�� ___ ����1�֣�
�ڽ�ÿֻС��ֱ����װ���У�װ�÷�����ͬ�Ļ����У���ʼʱ���������Һ��ȸߣ�ÿ��С���ϱ���ʾע��ҩ����
����A��ע��__ __����2�֣�
��10���Ӻ��¼���ձ���Һ�������߶ȣ�����____ ����2�֣�
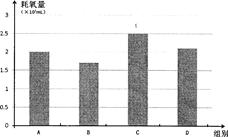
�� ��������� ��Ϊ����Ƚ��ϱ��и�����ֵ����ѡ������ͼ��ͼ(����ͼ)����2�֣�

�ڽ��������__ _�����ۣ�����2�֣�
�۳��˿��ƻ�����ͬ�⣬ʵ���������һ��������С��Ķ���װ�ã���Ŀ������_________���Ӷ�ʹ��õ���ֵ����ȷ����1�֣�
�� �ĵð����������ض�С�������(��������ʡ�����������)��Ӱ�� ��2�֣����� �� ����Ϊ���顣��1�֣���
�� A��ע��������ˮ��
�� �������������������ƽ��ֵ��
����
���ĵð����������طֱ���н��ͺ���ߺ����������ã������߹�ͬ���õ�ЧӦ�������(����)��
�����������������(����������)�������������仯(���������������ػ��������ض�ʵ������Ӱ�족����������������ػ�������ض�ʵ������Ӱ�족���ų��ر�����Ӱ�족�ɸ�1�֡���ͳ�𡰼�Сʵ���������֡�
��������
����������ɱ�ʵ��Ĺ��̿�֪ʵ���Ŀ����̽���ĵð����������ض�С�������(��������ʡ�����������)��Ӱ�� �� ע��ʵ������������ѭ��һ����ԭ��͵���ԭ��ʵ����Ҫ���з������ó����ۡ�ʵ�������Ҫ��ƺö������顣
���㣺ʵ�����
���������忼����ѧ����ʵ������������ѶȽϴ������Ĺؼ����������ʵ���ԭ��ע�ⲽ���������

�������غ͡��ĵð���ҩ�ﶼ����Ӱ������Ļ���ش�������⣺
��1���������ؼ���һ�ּ��أ�Ҳ��һ�����ʣ��ڹ��������������ڵ�_______���ӡ��ĵð����������ؾ������ƵĽṹ�����Ҷ���ֱ������������������ʣ��ɴ��Ʋ�����������ϸ��������ܾ�����ͬ�� ��
��2�����������غ��ĵð�����ʵ���о����±�Ϊ����ʵ������������ʵ�鱨�档
ʵ��Ŀ�ģ�̽��__________________________��
| ��� | ע��ҩ������ | ����������103mL�� | |
| �ĵð� | �������� | ||
| A | �� | �� | 2.0 |
| B | �� | �� | 1.7 |
| C | �� | �� | 2.5 |
| D | �� | �� | 2.1 |
| ע������������ע�䣬������������ע�䡣 | |||
�����þߣ�С������ֻ��ע��������������װ�ã�������ͼ��������������Һ���ĵð���Һ����������ˮ���ƣ���
�������裺
�� ѡȡ��С������״����ͬ��С��20ֻ������Ϊ���顣ÿ��С���ϱ���ʾע��ҩ��������A��ע��_______________����Ϊ___________��
�� ��ÿֻС��ֱ����װ���У���ʼʱ���ձ������Һ��ȸߡ�װ�÷�����ͬ�����У�10���Ӻ��¼_______________���������������������ƽ��ֵ��
���������Ϊ����Ƚ��ϱ��и�����ֵ����ѡ�����˵�ͼ�����ͻ�ͼ������ͼ����

ʵ����ۣ���__________________________________________________________
��_______________________________________________________
��12�֣��������غ͡��ĵð���ҩ�ﶼ����Ӱ������Ļ��ij��ȤС��Χ���������غ��ĵð��Ĺ��ܽ�����ʵ���о���������±���ʾ��������ʵ�鱨�沢�ش�������⣺��+��ʾ��ע�䣬����ʾû��ע�䡣��
| | ע��ҩ������ | | |
| ��� | �ĵð� | �������� | ������(��103mL) |
| A | - | - | 2��O |
| B | + | - | 1.7 |
| C | - | + | 2.5[ |
| D | + | + | 2.1 |
�� �����þߣ�С������ֻ��ע��������������װ��(������ͼ)������������Һ���ĵð���Һ(��������ˮ����)��

�� �������裺
��ѡȡ��С������״����ͬ��С��20ֻ�� ___ ����1�֣�
�ڽ�ÿֻС��ֱ����װ���У�װ�÷�����ͬ�Ļ����У���ʼʱ���������Һ��ȸߣ�ÿ��С���ϱ���ʾע��ҩ����
����A��ע��__ __����2�֣�
��10���Ӻ��¼���ձ���Һ�������߶ȣ�����____ ����2�֣�
�� ��������� ��Ϊ����Ƚ��ϱ��и�����ֵ����ѡ������ͼ��ͼ(����ͼ)����2�֣�

�ڽ��������__ _�����ۣ�����2�֣�
�۳��˿��ƻ�����ͬ�⣬ʵ���������һ��������С��Ķ���װ�ã���Ŀ������_________���Ӷ�ʹ��õ���ֵ����ȷ����1�֣�

