��Ŀ����
����������������ϸ���³´�л����绷��֮��Ĺ�ϵͼ���ش��������⡣

����(1)A����________��
����(2)B����ʳ������������գ��ɴ˿�ʹ������________����Ӫ���ɷֵ��Բ��䡣
����(3)������4������������C����________����ͨ��________ʵ�ֵġ�F����________�����徭��F��ѪҺ�ɷַ����ĸı���________��
����(4)G��������γɣ�������________��________�������̡������Һ�к���һ�����������ǣ���������������________�������䣬�����Һ�к��д����������ǣ�����________����________�������¡�
����(5)H���������µ��ڵĹ�ϵ��________��
����(6)�����ڵ���Ԫ�ֽ�������ǽ���������Ⱦ�________ѭ������________ѭ�������͵�ȫ������֯ϸ���б������ֽ⣬���е�________��ϳ�________ͬʱ�ͷ�������������һ����ת�Ƹ�________������________������������������Ҫʱ���á�
����(7)�������ڸ����ھ��Ѱ��������������İ�������ת��Ϊ________����[����]________�ų����⡣
����(8)���ܰ�ϵͳ�У�����С�����ܰ��ڵ��ܰ��������ܰͲ�ͬ����________��
����(9)ѭ��ϵͳ������������壬����[����]________ëϸѪ��ʵ�����彻�������彻���Ķ�����________��
������
|
(1)��֯ϸ���� 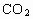 �϶�ľ���Ѫ��ɺ�O2�Ϸḻ���� �϶�ľ���Ѫ��ɺ�O2�Ϸḻ����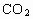 ���ٵĶ���Ѫ�� ���ٵĶ���Ѫ��(4)��С����˹�������С�ܵ����������ã���С�ܣ��ȵ����ȵ��أ� (5)��Һ��������������϶����������Һ���ڶ࣬�����࣬��������ɢʧ�Ͷ�Щ���෴����������ɢʧ����һЩ�� (6)�Σ��壬�������ˮ��ADP��ATP�� (7)���أ�G�����ࣻ (8)���н϶��֬�����ʣ� (9)E�����ݣ������ѹ���� |

��ÿ��1�֣���14�֣�
����ͻ�������ù�����ڻ����������������ʵ��İ�������������������������ͣ����������������������ͺ�ɫ���ù��ͻ����a��ͻ����b����������ʵ�飺
ʵ��һ������������ͻ����ֱ����������6���������ϣ�����ͻ���겻����l��3��5��
����������������2��4��6�������϶����������������ijɷ����±���ʾ����ͻ����a��
b��Ҫ����A��J�е����������������������
| �������� | �����ɷ� | ���ӵİ��������� |
| �� | | �� |
| �� | | �����¡��á��� |
| �� | ���������� | �¡��š��ơ��� |
| �� | �á��ơ��ȡ��� | |
| �� | �ġ��ǡ��ɡ��� | |
| �� | �����ʶ��� |
��ע��������������Ұ���;��������������������
ʵ������ٶ�ͻ����a��b����İ�����Ϊ�أ���Ұ�������ڣ�����ͼ��ʾ��;���ϳɣأ���Ӧ�١��ڡ��۷ֱ��ɲ�ͬ��ø�����������ӹϰ���ʱ��ͻ����a��������������������������������ӹϰ��ỹ�����ᣬͻ����b������������
����������Ӧ�� ��Ӧ�� ��Ӧ��


 ���� �������� �ϰ��� ����������X
���� �������� �ϰ��� ����������Xͻ����a����������ԭ���������������裬ͻ����b�����������������裬��Ӧ����ĸ���ԭ��������������������������������
ʵ������������Y��������Ҫ���϶�İ����ᣬΪʹ���ù���ڴ�л�����в�������İ�����Y��������ͼ�жϡ�
ø�� ø�� ø��


 ���� ���� �ϰ��� ������X
���� ���� �ϰ��� ������X
ø��
������Y
Ӧ�ö����ù�п��������������ϳɵĻ�������ձ��ƻ����ձ�֮ǰ���Ŵ���Ϣ�Ĵ���;��������������������������������������
���ش������й���������⣺
������ͼһΪijϸ���ϳ�ij�ְ�����Ĵ�л����ʾ��ͼ�����ұ�����ʾ�м�����
|

�������л�ĵ���������ø����________ø����������ϸ���л��۹��࣬������ø��Ļ��ԣ����ֵ��ڷ�ʽ�����������������������������ص㡣
��2���±���ij�����������ɷ֡�����ݴ˱��ش��������⣺
| ��� | �� | �� | �� | �� | �� | �� | �� |
| �ɷ� | S | ��NH4��2SO4 | K2HPO4 | MgSO4 | FeSO4 | CaCl2 | H2O |
| ���� | 10g | 0��4g | 4��0g | 9��25g | 0��5g | 0��5g | 100mL |
��ijͬѧ�ô��������ֱ���������ͽ�ĸ������������װ�÷��ڹ��������������ⶨ����Һ������ͽ�ĸ����������������Ƴ�����ͼ������ͬ���������ͷ�����������������������������ԭ����������������������������

��Ŀǰͨ�������̿����������������ȵ��ء�ͼ����ʾ�������ȵ��ػ���Ĵ˾��������ߣ�����BC������________�ڣ�����AC�δ˾���Ⱥ�����仯��________���������˾���л����ȵ��أ�����ʱ���൱�����ߵ�________�Σ���ϸ�����ֶ�
����̬����������ʱ���˾�Ⱥ���������뵽ͼ�е�________�Ρ�
 ���еĹ�������֬��ϸ����DDT�ĺ����ﵽ30.2��
���еĹ�������֬��ϸ����DDT�ĺ����ﵽ30.2�� (dioxin)������ʹ��������Ⱦ�����ϣ�������ּ�֬���ж��f
(dioxin)������ʹ��������Ⱦ�����ϣ�������ּ�֬���ж��f


