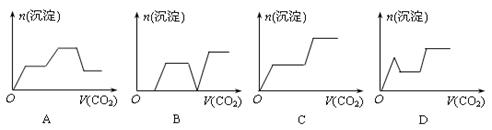
��Ŀ����
����������ȷ���ǣ� ��
| A������ʱ��ij��Һ����ˮ���������c��H������c��OH�����ij˻�Ϊ1��10��24������Һ��һ�����Դ�������K����Na����AlO2����SO42�� |
| B������ʱ��0.1mol/L HA��Һ��pH��1��0.1mol/L BOH��Һ��c��OH����/c��H����=1012������������Һ�������ϣ���Ϻ���Һ������Ũ�ȵĴ�С��ϵΪ��c��B������c��OH������c��H������c��A���� |
| C������SO2ͨ�뵽Ba��NO3��2��Һ�У���ȷ�����ӷ�Ӧ����ʽΪ��3SO2��2NO3����3Ba2����2H2O=3BaSO4����2NO����4H�� |
| D�������£�ϡ��0.1 mol��L�İ�ˮ����Һ��c��OH-����c��NH4+����c��H+�����½� |
C
�������������A. ����ʱˮ�����ӻ�������1��10��14��������Һ����ˮ���������c��H������c��OH�����ij˻�Ϊ1��10��24��ˮ�ĵ����ܵ������ơ�˵������Һ��������������������ʱAlO2�����ܴ������ڡ�����B������ʱ��0.1mol/L HA��Һ��pH��1����HAΪ���0.1mol/L BOH��Һ��c��OH����/c��H����=1012��C��OH������c��H����=10-14�����c��OH-��=0.1mol/L.˵��BOH��ǿ�����������Һ�������ϣ�ǡ����ȫ��Ӧ����ǿ��������BA��A-ˮ��ʹ��Һ�Լ��ԣ�C(B+)>C(A -);C(OH-)>C(H+)��������ˮ��ij̶��Ǻ����ģ�����C(A -)>C(OH-)���ʣ���Ϻ���Һ������Ũ�ȵĴ�С��ϵΪ��c��B������c��A������c��OH������c��H����������C��SO2����ˮ���������ᣬ������������������������������������ã������������������ᡣ����������뱵���ӽ�ϳ����ᱵ���������Խ�����SO2ͨ�뵽Ba��NO3��2��Һ�У���ȷ�����ӷ�Ӧ����ʽΪ��3SO2��2NO3����3Ba2����2H2O=3BaSO4����2NO����4H����ȷ��D����ˮ�д��ڵ���ƽ�⣺NH3��H2O NH4++OH-������ˮϡ��ʱ������ƽ���������ƶ���c��OH-����c��NH4+����С�������ڳ�������Һ��c��H+����c��OH-���Ǹ�����������c��H+��������
NH4++OH-������ˮϡ��ʱ������ƽ���������ƶ���c��OH-����c��NH4+����С�������ڳ�������Һ��c��H+����c��OH-���Ǹ�����������c��H+��������
���㣺�����������Һ������Ũ�ȵıȽϡ�ˮ�����ӻ�������Ӧ�ú�������ԭ��Ӧ�ȵ�֪ʶ��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����ȷ��ʾ���з�Ӧ�����ӷ���ʽΪ
| A��̼��������Һ�е�������������Һ��HCO3-+OH�C= CO32-+ H2O |
| B����������ͨ�����������Һ��SO2 + ClO- + 2OH�C= SO42-+Cl-+ H2O |
| C��������ϡ���BaS + 2H+ = H2S��+ Ba2+ |
| D����KIO3����������Һ�е�KI��5I����IO3����3H2O="3I" 2��6OH�� |
����������ȷ����
A��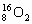 �� �� ��Ϊͬλ�أ��������� ��Ϊͬλ�أ��������� |
| B�������£�pH=1��ˮ��Һ��Na+��NO3-��HCO3-��Fe2+���Դ������� |
| C��������Ư�۳���������ˮ�ľ�����ɱ�����������ߵ�����ԭ����ͬ |
| D��C(ʯī��s)=C(���ʯ��s)��H��0������ʯī�Ƚ��ʯ�ȶ� |
ij��Һ���ܺ���Cl-��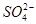 ��
�� ��NH+ 4��Fe3+��Al3+��K+��ȡ����Һ100 mL���������NaOH��Һ�����ȣ��õ�0.02 mol���壬ͬʱ�������ɫ���������ˣ�ϴ�ӣ����գ��õ�1.6 g���壻��������Һ�м�����BaCl2��Һ���õ�4.66 g����������ij������ɴ˿�֪ԭ��Һ��
��NH+ 4��Fe3+��Al3+��K+��ȡ����Һ100 mL���������NaOH��Һ�����ȣ��õ�0.02 mol���壬ͬʱ�������ɫ���������ˣ�ϴ�ӣ����գ��õ�1.6 g���壻��������Һ�м�����BaCl2��Һ���õ�4.66 g����������ij������ɴ˿�֪ԭ��Һ��
| A��NH+ 4Fe3+һ������, K+���ܴ��� |
| B��Cl?һ�����ڣ���c(Cl?)��0.4 mol/L |
C��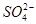 ��NH+ 4Fe3+һ�����ڣ������ʵ���Ũ�Ⱦ�Ϊ0.02 mol/L ��NH+ 4Fe3+һ�����ڣ������ʵ���Ũ�Ⱦ�Ϊ0.02 mol/L |
D�� ��Al3+һ�������ڣ�K+һ������ ��Al3+һ�������ڣ�K+һ������ |
ij��Һ�п��ܺ���Na����Ca2����Br����CO32����I����SO32�����������еļ��֡����ڸ���Һ�еμ�������ˮ�������ݲ�������Һ�ʳȻ�ɫ������ʳȻ�ɫ����Һ�м���BaCl2��Һʱ�������ɣ��۳Ȼ�ɫ��Һ����ʹ������Һ������������ʵ����ʵ�ƶϣ��ڸ���Һ�п϶����ڵ��������� ( )
| A��Na����Br����CO32�� | B��Na����SO32����I�� |
| C��Ca2����I����SO32�� | D��Ca2����CO32����Br�� |
���������к��������ƶ���Cl�D���ǣ� ��
| A��KClO3��Һ | B��Һ̬HCl |
| C��KCl��Һ | D��NaCl���� |
���������У��ܹ����������ڵ���ʵ��ǣ� ��
| A��Cu˿ | B�����ڵ�MgCl2 | C��ϡ���� | D�������Ȼ��� |
���и������ʣ�ǰ�����ڵ���ʣ��������ڷǵ���ʵ��ǣ� ��
| A��NaCl���塢BaSO4 | B��ͭ���������� |
| C��Һ̬�Ĵ��ᡢ�ƾ� | D�����ڵ�KNO3��������Һ |