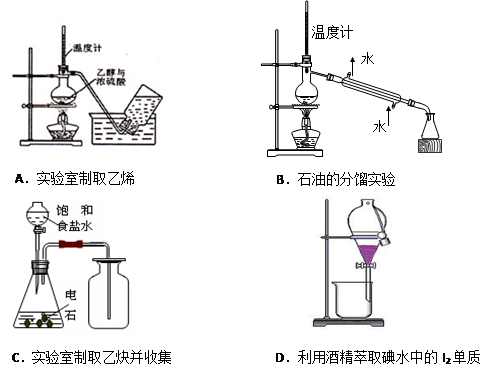
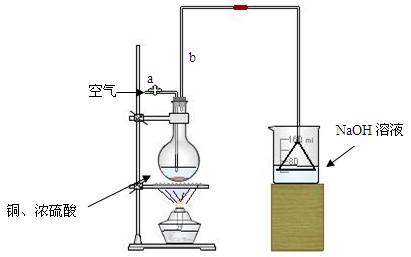
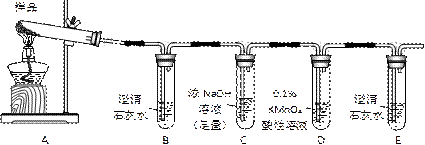��Ŀ����
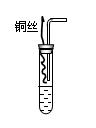
��12�֣����ӷϾɵ�ػᵼ�����صĻ�����Ⱦ��һ�ڷϵ�ؾ���һ�š�ը������ij��ѧ��ȤС���ͬѧ��̽���ϸɵ���ڵĺ�ɫ�����������ʱ����������ͼ��ʾ��ʵ�飺

���Ľ̲Ŀɵõ�������Ϣ��
����ͨп�̵�صĺ�ɫ������Ҫ�ɷ�ΪMnO2��NH4Cl��ZnCl2�����ʡ�
��Zn��OH��2���ܽ��ڹ����İ�ˮ�С�
��ش��������⣺
��1����������������ʱ���õ�����Ҫ�����оƾ��ƣ��������� �������Ǻ����żܣ����������еĺ�ɫ����ʱ������һ��ʹ�����ʯ��ˮ����ǵ����壬�ɴ��Ʋ������л�����̼��
��2�������ܵ��Թ��м���������ú�ɫ�������Թ���Ѹ�ٲ�����ʹ�����ǵ�ľ����ȼ�����壬�ݴ˿ɳ����϶���ɫ����Ϊ ��
��3����ͬѧ����Һ�ijɷֽ��м��飬��ȷ���Ƿ���NH4Cl��ZnCl2��������������ʵ�����д��ʵ�鱨�棬����д����հ״������ݣ�
��4����������ʵ�鱨�棬������Һ�ijɷ֣�ͬѧ�ǵĽ����ǣ���Һ�к����Ȼ�狀��Ȼ�п���������Һ�еõ����ʹ��壬��Ӧ���еò����� ����Ҫ�����õ����ʹ����е����ʼ��Է��룬���� ����


���Ľ̲Ŀɵõ�������Ϣ��
����ͨп�̵�صĺ�ɫ������Ҫ�ɷ�ΪMnO2��NH4Cl��ZnCl2�����ʡ�
��Zn��OH��2���ܽ��ڹ����İ�ˮ�С�
��ش��������⣺
��1����������������ʱ���õ�����Ҫ�����оƾ��ƣ��������� �������Ǻ����żܣ����������еĺ�ɫ����ʱ������һ��ʹ�����ʯ��ˮ����ǵ����壬�ɴ��Ʋ������л�����̼��
��2�������ܵ��Թ��м���������ú�ɫ�������Թ���Ѹ�ٲ�����ʹ�����ǵ�ľ����ȼ�����壬�ݴ˿ɳ����϶���ɫ����Ϊ ��
��3����ͬѧ����Һ�ijɷֽ��м��飬��ȷ���Ƿ���NH4Cl��ZnCl2��������������ʵ�����д��ʵ�鱨�棬����д����հ״������ݣ�
| ʵ��Ŀ�� | ���� | ʵ������ | ���� |
| ����Cl�� | ȡ������Һ���Թ��У� | | ����Cl�� |
| ����NH4�� | ȡ������Һ���Թ��У� | | ����NH4�� |
| ����Zn2�� | ȡ������Һ���Թ��У� | | ����Zn2�� |
��1������ ��1�֣� ��2���������̣���MnO2�� ��2�֣�
��3����ÿ��1�֣�
��4���ڻ��Һ�м����������ᣬ��������������Ũ��������ȴ�ᾧ�����ˣ�2�֣�
���ȣ�1�֣�
��3����ÿ��1�֣�
| ���� | ʵ������ |
| ���������ữ����������Һ | �а�ɫ�������� |
| ����Ũ����������Һ�����ȣ�����ʪ�ĺ�ɫʯ����ֽ�����Թܿڸ��� | ��ɫʯ����ֽ�����ɫ |
| ϡ��ˮ | �Ȳ�����ɫ������������ȥ��ˮ�������ܽ� |
���ȣ�1�֣�
��

��ϰ��ϵ�д�
 �Ͻ�ƽ��У����ϵ�д�
�Ͻ�ƽ��У����ϵ�д�
�����Ŀ