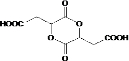
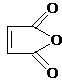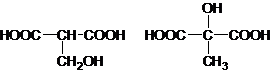��Ŀ����
�밴Ҫ��ش��������⣺
��1���������ǽ���������һֱ��������Ҫ��ɫ������Ϊ���ǽ������ϵ����ǡ�
��������Ʒ�õ��赥�ʵ��� ��
A��������Ʒ B��ʯӢ�ӱ� C�������оƬ D�����ά E��̫���ܵ��
�� ��������ɸ��ӣ����������������ʽ��ʾ��ij����������Ҫ��ѧ�ɷ�ΪCa2Mg5Si8O22(OH)2. ����д�����������ʽΪ�� ��
��2��Cl2��SO2������Ư���ԣ��������������尴�����1:1ͨ��ˮ�еõ�����Һȴ����û��Ư���ԣ�д��Cl2��SO2 1:1ͨ��ˮ�з��������ӷ�Ӧ����ʽ��_______________________________________________________________________��
��3��д������������˫��ˮ��Ӧ�Ļ�ѧ����ʽ�����������ת�Ʒ������Ŀ�� ______________________ ________ ____ ��
��1��C��E (2��) 2CaO��5MgO��8SiO2��H2O ��2�֣�
��2��Cl2 + SO2 + 2H2O == 2Cl- +SO42- + 4H+ ��2�֣�
(3) H2O2 + SO2 ==H2SO4 ������ʽ��˫���Ż����Ÿ�2�֣�

 �ٷ�ѧ����ҵ��������ϵ�д�
�ٷ�ѧ����ҵ��������ϵ�д�