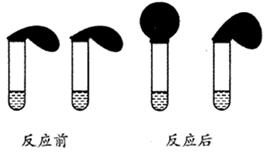
ĢāÄæÄŚČŻ
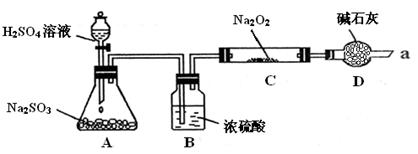
ĮņĖįŠææÉ×÷ĪŖŹ³Ę·ŠæĒæ»Æ¼ĮµÄŌĮĻ”£¹¤ŅµÉĻ³£ÓĆĮāŠææóÉś²śĮņĖįŠæ£¬ĮāŠææóµÄÖ÷ŅŖ³É·ÖŹĒZnCO3£¬²¢ŗ¬ÉŁĮæµÄFe2O3 ”¢FeCO3 ”¢MgOµČ£¬²æ·ÖÉś²ś¹¤ŅÕĮ÷³ĢĶ¼Ź¾ŅāČēĻĀ£ŗ
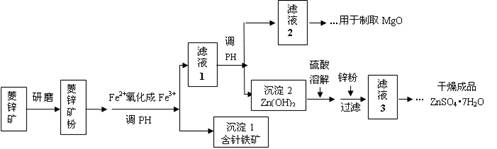
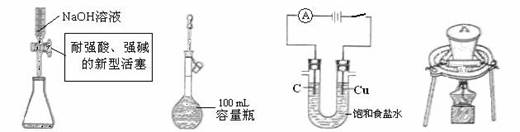
£Ø1£©½«ĮāŠææóŃŠÄ„³É·ŪµÄÄæµÄŹĒ ”£
£Ø2£©¹¤ŅµĮ÷³ĢÖŠ±ŲŠė½«Fe2+Ńõ»Æ³ÉFe3+ŗóŌŁ¼ÓŅŌ·ÖĄė”£ŌŚŹµŃéŹŅÖŠæÉŅŌÓĆH2O2£¬ŌŚĖįŠŌĢõ¼žĻĀĶź³ÉÕāøö×Ŗ»Æ£¬ĒėŠ“³öĻąÓ¦µÄĄė×Ó·½³ĢŹ½£ŗ
ӣ
£Ø3£©ÕėĢśæóµÄ×é³ÉŌŖĖŲŹĒFe”¢OŗĶH£¬»ÆѧŹ½ĮæĪŖ89£¬»ÆѧŹ½ŹĒ ”£
£Ø4£©¹¤ŅµÉĻ“Ó”°ĀĖŅŗ2”±ÖĘČ”MgO¹ż³ĢÖŠ£¬ŗĻŹŹµÄ·“Ó¦ĪļŹĒ £ØŃ”ĢīŠņŗÅ£©”£
a£®“óĄķŹÆ·Ū b£®ŹÆ»ŅČé c£®“æ¼īČÜŅŗ d£®ÉÕ¼īČÜŅŗ
£Ø5£©”°ĀĖŅŗ3”±Ö®ŗóµÄ²Ł×÷ŅĄ“ĪĪŖ ”¢ ”¢¹żĀĖ£¬Ļ“µÓ£¬øÉŌļ”£

£Ø1£©½«ĮāŠææóŃŠÄ„³É·ŪµÄÄæµÄŹĒ ”£
£Ø2£©¹¤ŅµĮ÷³ĢÖŠ±ŲŠė½«Fe2+Ńõ»Æ³ÉFe3+ŗóŌŁ¼ÓŅŌ·ÖĄė”£ŌŚŹµŃéŹŅÖŠæÉŅŌÓĆH2O2£¬ŌŚĖįŠŌĢõ¼žĻĀĶź³ÉÕāøö×Ŗ»Æ£¬ĒėŠ“³öĻąÓ¦µÄĄė×Ó·½³ĢŹ½£ŗ
ӣ
£Ø3£©ÕėĢśæóµÄ×é³ÉŌŖĖŲŹĒFe”¢OŗĶH£¬»ÆѧŹ½ĮæĪŖ89£¬»ÆѧŹ½ŹĒ ”£
£Ø4£©¹¤ŅµÉĻ“Ó”°ĀĖŅŗ2”±ÖĘČ”MgO¹ż³ĢÖŠ£¬ŗĻŹŹµÄ·“Ó¦ĪļŹĒ £ØŃ”ĢīŠņŗÅ£©”£
a£®“óĄķŹÆ·Ū b£®ŹÆ»ŅČé c£®“æ¼īČÜŅŗ d£®ÉÕ¼īČÜŅŗ
£Ø5£©”°ĀĖŅŗ3”±Ö®ŗóµÄ²Ł×÷ŅĄ“ĪĪŖ ”¢ ”¢¹żĀĖ£¬Ļ“µÓ£¬øÉŌļ”£
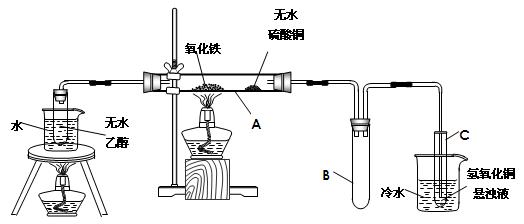
£Ø1£©Ōö“ó·“Ó¦Īļ½Ó“„Ć껿»ņŌö“ó·“Ó¦ĖŁĀŹ»ņŹ¹·“Ó¦øü³ä·Ö
£Ø2£©2Fe2++ H2O2+2H+ ="==" 2H2O +2Fe3+
£Ø3£©FeO£ØOH£©£Ø»ņĘäĖüŗĻĄķ“š°ø£©
£Ø4£©b»ņ£ØbŗĶd£©£»d
£Ø5£©Õō·¢ÅØĖõ”¢ĄäČ“½į¾§£Ø»ņĘäĖüŗĻĄķ“š°ø£©
£Ø2£©2Fe2++ H2O2+2H+ ="==" 2H2O +2Fe3+
£Ø3£©FeO£ØOH£©£Ø»ņĘäĖüŗĻĄķ“š°ø£©
£Ø4£©b»ņ£ØbŗĶd£©£»d
£Ø5£©Õō·¢ÅØĖõ”¢ĄäČ“½į¾§£Ø»ņĘäĖüŗĻĄķ“š°ø£©
ĀŌ

Į·Ļ°²įĻµĮŠ“š°ø
Ļą¹ŲĢāÄæ

 L-1BaCl2Ӣ2mol
L-1BaCl2”¢2mol ²½Öč1£ŗ·Ö±šČ”ÉŁĮæ·“Ó¦ŗóCÖŠµÄ¹ĢĢåÖĆÓŚA”¢BŹŌ¹ÜÖŠ”£
²½Öč1£ŗ·Ö±šČ”ÉŁĮæ·“Ó¦ŗóCÖŠµÄ¹ĢĢåÖĆÓŚA”¢BŹŌ¹ÜÖŠ”£