��Ŀ����
[���ʽṹ������] ����ǰ������A��B��C��D��E��X����Ԫ�أ���֪B��C��D��E��A���ַǽ���Ԫ��ԭ�Ӱ뾶���μ�С������B��s�ܼ��ϵ�����������p�ܼ��ϵ���������2����Xԭ�ӵ�M�ܲ�����4��δ�ɶԵ��ӡ���ش��������⣺
��1��д��C��D��E����ԭ�ӵ�һ�������ɴ�С��˳��Ϊ_______________��
��2��Aԭ����B��C��Dԭ���γ����������ȶ�����ǿ������˳��Ϊ_____________�����ݼ۲���ӶԻ�������Ԥ��BA2D�ķ��ӹ���Ϊ____________��
��3��ij��ɫ���壬��ṹ�ص���X2+��X3+���ӷֱ�ռ�������廥�����ڵĶ��㣬���������ÿ�����Ͼ���һ��BC-����Aͬ��������������ڵ�Ԫ��R������λ���������ǡ��λ���ϡ�������ṹ�ص��֪�þ���Ļ�ѧʽΪ���������������ʾ��________
��4����ѧ��ͨ��X����̽����KCl��MgO��CaO��TiN�ľ���ṹ��NaCl�ľ���
�ṹ���ƣ�����ͼ��ʾ��������3�����Ӿ���ľ������������±���

| ���Ӿ��� | NaCl | KCl | CaO |
| ������/kJ��mol��1 | 786 | 715 | 3401 |
���ݱ����е����ݣ��ж�KCl��MgO��TiN �������Ӿ����۵�Ӹߵ��͵�˳����______________��MgO������һ��Mg2����Χ�������ڽ��ҵȾ����O2-��__________________����
��5���о����ʴ��Ա��������������Ӻ�δ�ɶԵ���Խ�࣬�����Խ�ż�¼����Խ�á�������������V2O5��Cr2O3�У��ʺ���¼�����ŷ�ԭ�ϵ���__________________��
��1��F��N��O��2�֣���2��H2O��NH3��CH4��2�֣�����ƽ�棩�����Σ�2�֣�
��3��NaFe2(CN)6��3�֣���4��TiN��MgO��KCl ��2�֣� 6 ��2�֣���5��Cr2O3��2�֣�

 ��2013?����һģ��[��ѧ--ѡ��3���ʽṹ������]
��2013?����һģ��[��ѧ--ѡ��3���ʽṹ������]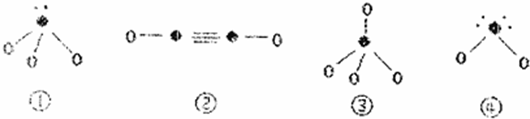
 [��ѧ-ѡ�����ʽṹ������]����A��B��C��D��E��F�������ʻ����ӣ�����A��B��C��D��������ͼ��ʾ�Ľṹ��ṹ��Ԫ��ͼ����������еIJ���δ���������߲���ʾ��ѧ������Ӽ���������X��Y������ͬҲ���Բ�ͬ����A��B�ľ���������ͬ������A��ͬ������������B���ʷ����û���Ӧ��C��D��E��F������ͬ�ĵ���������D�������ӣ�D��F�����Ԫ����ͬ��C��E��F�ľ���������ͬ����E���ɵ����ʳ����³�Һ̬��
[��ѧ-ѡ�����ʽṹ������]����A��B��C��D��E��F�������ʻ����ӣ�����A��B��C��D��������ͼ��ʾ�Ľṹ��ṹ��Ԫ��ͼ����������еIJ���δ���������߲���ʾ��ѧ������Ӽ���������X��Y������ͬҲ���Բ�ͬ����A��B�ľ���������ͬ������A��ͬ������������B���ʷ����û���Ӧ��C��D��E��F������ͬ�ĵ���������D�������ӣ�D��F�����Ԫ����ͬ��C��E��F�ľ���������ͬ����E���ɵ����ʳ����³�Һ̬��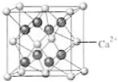 ��4��B��Ca�γɵľ���ľ�������ͼ��ʾ������Ca2+����λ����
��4��B��Ca�γɵľ���ľ�������ͼ��ʾ������Ca2+����λ����