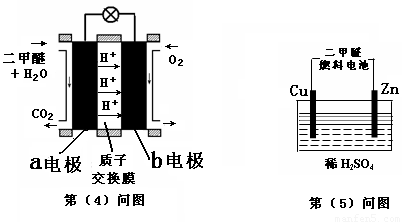
��Ŀ����
 ����������һ�ֽྻ������������Դ��������������Ҫ�ɷ�ΪCO��CO2��H2�ȣ���H2��ϣ����ϳɼ״��������������õķ���֮һ��
����������һ�ֽྻ������������Դ��������������Ҫ�ɷ�ΪCO��CO2��H2�ȣ���H2��ϣ����ϳɼ״��������������õķ���֮һ����1��������Ӧ�Ĵ�������Cu��Zn��Al��Ԫ�أ�д����̬Znԭ�ӵĺ�������Ų�ʽ
1s22s22p63s23p63d104s2��[Ar]3d104s2
1s22s22p63s23p63d104s2��[Ar]3d104s2
����2�����ݵȵ���ԭ����д��CO���ӽṹʽ
C��O
C��O
����3���״��������ɵõ���ȩ����ȩ������Cu��OH��2�ļ�����Һ��Ӧ����Cu2O������
�ټ״��ķе�ȼ�ȩ�ĸߣ�����Ҫԭ����
�״�����֮���γ����
�״�����֮���γ����
����ȩ������̼ԭ�ӹ�����ӻ�����Ϊsp2�ӻ�
sp2�ӻ�
���ڼ�ȩ���ӵĿռ乹����
ƽ��������
ƽ��������
��1mol��ȩ�����ЦҼ�����ĿΪ3NA
3NA
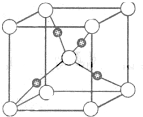
������1��Cu2O�����У��ṹ��ͼ1��ʾ������������Cuԭ����ĿΪ
4
4
����4��Ԫ��Cu��һ���Ȼ��ᄃ��ľ����ṹ��ͼ2��ʾ�����Ȼ���Ļ�ѧʽ��
CuCl
CuCl
��������Ũ���ᷢ����������ԭ��Ӧ�����������HnCuCl3����Ӧ�Ļ�ѧ����ʽΪCuCl+2HCl=H2CuCl3
CuCl+2HCl=H2CuCl3
����������1�����ݹ���ԭ����дZn�ĵ����Ų�ʽ��
��2��CO��N2Ϊ�ȵ����壬����N2�Ľṹ�жϣ�
��3���ټ״��к���������е�ϸߣ�����ԭ���γ�3���ļ���
�ڸ��ݼ۲���ӶԻ���ģ���жϣ�
�������ݾ���ʾ��ͼ���Կ���Cuԭ�Ӵ��ھ����ڲ����Դ��ж�ͭԭ������
��4�����þ�̯�����㾧���Ľṹ����������غ㶨����д��ѧ����ʽ��
��2��CO��N2Ϊ�ȵ����壬����N2�Ľṹ�жϣ�
��3���ټ״��к���������е�ϸߣ�����ԭ���γ�3���ļ���
�ڸ��ݼ۲���ӶԻ���ģ���жϣ�
�������ݾ���ʾ��ͼ���Կ���Cuԭ�Ӵ��ھ����ڲ����Դ��ж�ͭԭ������
��4�����þ�̯�����㾧���Ľṹ����������غ㶨����д��ѧ����ʽ��
����⣺��1��Zn��ԭ������Ϊ30��ע��3d���д��4s�����ǰ�棬�����Ų�ʽΪ1s22s22p63s23p63d104s2��[Ar]3d104s2��
�ʴ�Ϊ��1s22s22p63s23p63d104s2��[Ar]3d104s2��
��2�����ݵȵ���ԭ������֪CO��N2Ϊ�ȵ����壬N2���ӵĽṹʽΪ��N��N����Ϊ�ȵ�������ӵĽṹ���ƣ���д��CO�ĽṹʽΪC��O���ʴ�Ϊ��C��O��
��3���ټ״�����֮���γ��˷��Ӽ��������ȩ���Ӽ�ֻ�Ƿ��Ӽ�����������û���γ�������ʼ״��ķе�ߣ���ȩ�����к���̼��˫������̼ԭ�ӹ�����ӻ�����Ϊsp2�ӻ����ʴ�Ϊ���״�����֮���γ������sp2�ӻ���
�ڼ�ȩΪsp2�ӻ��������µ��Ӷԣ����ӵĿռ乹��Ϊƽ�������Σ�1mol��ȩ�����к���2mol̼��ļ���1mol̼���ļ����ʺ��Цļ������ʵ���Ϊ3mol����ĿΪ3NA����
�ʴ�Ϊ��ƽ�������Σ�3NA��
�����ݾ���ʾ��ͼ���Կ���Cuԭ�Ӵ��ھ����ڲ�����������Cuԭ����ĿΪ4���ʴ�Ϊ��4��
��4���ɾ�����֪Cuԭ��λ�ھ����ڲ�������4��Cu��Clλ�ڶ�������ģ�������8��
+6��
=4�����Ȼ���Ļ�ѧʽ��CuCl��������Ũ���ᷢ����������ԭ��Ӧ�����������HnCuCl3����Ӧ��Ҫ2HCl��
��Ӧ�Ļ�ѧ����ʽΪCuCl+2HCl=H2CuCl3��
�ʴ�Ϊ��CuCl��CuCl+2HCl=H2CuCl3��
�ʴ�Ϊ��1s22s22p63s23p63d104s2��[Ar]3d104s2��
��2�����ݵȵ���ԭ������֪CO��N2Ϊ�ȵ����壬N2���ӵĽṹʽΪ��N��N����Ϊ�ȵ�������ӵĽṹ���ƣ���д��CO�ĽṹʽΪC��O���ʴ�Ϊ��C��O��
��3���ټ״�����֮���γ��˷��Ӽ��������ȩ���Ӽ�ֻ�Ƿ��Ӽ�����������û���γ�������ʼ״��ķе�ߣ���ȩ�����к���̼��˫������̼ԭ�ӹ�����ӻ�����Ϊsp2�ӻ����ʴ�Ϊ���״�����֮���γ������sp2�ӻ���
�ڼ�ȩΪsp2�ӻ��������µ��Ӷԣ����ӵĿռ乹��Ϊƽ�������Σ�1mol��ȩ�����к���2mol̼��ļ���1mol̼���ļ����ʺ��Цļ������ʵ���Ϊ3mol����ĿΪ3NA����
�ʴ�Ϊ��ƽ�������Σ�3NA��
�����ݾ���ʾ��ͼ���Կ���Cuԭ�Ӵ��ھ����ڲ�����������Cuԭ����ĿΪ4���ʴ�Ϊ��4��
��4���ɾ�����֪Cuԭ��λ�ھ����ڲ�������4��Cu��Clλ�ڶ�������ģ�������8��
| 1 |
| 8 |
| 1 |
| 2 |
��Ӧ�Ļ�ѧ����ʽΪCuCl+2HCl=H2CuCl3��
�ʴ�Ϊ��CuCl��CuCl+2HCl=H2CuCl3��
�����������ԭ�ӽṹ�����ӽṹ������ṹ�Ϻõ��ں���һ���ۺϿ��������ʽṹ�����ʵ�����֪ʶ���ڵڣ�3������оͿ�����Ӱ����Ӿ���е�ߵ͵����أ����龧���֪ʶ�㶼�Ƚϼ��������ʽҲ������յ���ʽ���ֵģ����ֻҪ����ƽʱ���ڹ����ж��жϾ������͵ķ���������Ӱ�쾧�����ʵ����أ�ͬʱѧ��������á���̯����������������ļ��ɼ��ɣ���Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ

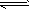 CO2(g)+H2(g)���÷�Ӧ�ġ�H________0��ѡ�����������������������������900Kʱ���÷�Ӧƽ�ⳣ���Ķ���ֵ��lgK��=_____________.
CO2(g)+H2(g)���÷�Ӧ�ġ�H________0��ѡ�����������������������������900Kʱ���÷�Ӧƽ�ⳣ���Ķ���ֵ��lgK��=_____________.