题目内容
已知:Fe2O3 (s)+C(s)= CO2(g)+2Fe(s) △H=234.1kJ·mol-1
C(s)+O2(g)=CO2(g) △H=-393.5kJ·mol-1 则2Fe(s)+O2(g ) = Fe2O3(s) 的△H是
则2Fe(s)+O2(g ) = Fe2O3(s) 的△H是
C(s)+O2(g)=CO2(g) △H=-393.5kJ·mol-1
 则2Fe(s)+O2(g ) = Fe2O3(s) 的△H是
则2Fe(s)+O2(g ) = Fe2O3(s) 的△H是| A.-824.4kJ·mol-1 | B.-627.6kJ·mol-1 |
| C.-744.7kJ·mol-1 | D.-169.4kJ·mol-1 |
A
略

练习册系列答案
 名校课堂系列答案
名校课堂系列答案
相关题目
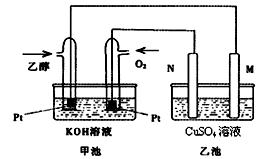
 下),理论上需通入乙醇 g?
下),理论上需通入乙醇 g?

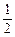 O2(g)=CO2(g) ΔH3<0 ③
O2(g)=CO2(g) ΔH3<0 ③  中和热为57.3kJ/mol 。下列热化学方程式正确的是
中和热为57.3kJ/mol 。下列热化学方程式正确的是 -57.3kJ/mol.
-57.3kJ/mol.