��Ŀ����
������и�С���еĻ�ѧ��Ӧ����ʽ��
�ż������O2��Ӧ����������ϸ��ӣ�����ͨ�������Na2O�����������Na2O2�����г�������ͳ�������ȡ����Ҫ�Ʊ����������ͨ������һ�����ü������ԭ��Ӧ�Ĺ�����������λ��������Ρ����û�ѧ����ʽ��ʾ���·�Ӧ��������������Ʒ�Ӧ ��
�ڼػ�ԭ����ػ�������һ�ֵ������� ��
�����ܴ������������ж�ȡ�����������������Գ�����ұ�����۽����⣬��������ȡ���µĽ����մɡ����罫���ۡ�ʯī�Ͷ������Ѱ�һ��������;��ȣ����ڽ��������ϣ�Ȼ���ڸ��������գ��������ȵ�TiC��д���÷�Ӧ�Ļ�ѧ����ʽ ��
�ǽ�Al(OH)3��Na2CO3�Ļ����һͬ�����������Ƶõ�ⷨ����������ۼ�����ʯ��Na3AlF6����ͬʱ��Ӧ�в���һ����ɫ��ζ�����壬��д���йػ�ѧ����ʽ_________________��
�ż������O2��Ӧ����������ϸ��ӣ�����ͨ�������Na2O�����������Na2O2�����г�������ͳ�������ȡ����Ҫ�Ʊ����������ͨ������һ�����ü������ԭ��Ӧ�Ĺ�����������λ��������Ρ����û�ѧ����ʽ��ʾ���·�Ӧ��������������Ʒ�Ӧ ��
�ڼػ�ԭ����ػ�������һ�ֵ������� ��
�����ܴ������������ж�ȡ�����������������Գ�����ұ�����۽����⣬��������ȡ���µĽ����մɡ����罫���ۡ�ʯī�Ͷ������Ѱ�һ��������;��ȣ����ڽ��������ϣ�Ȼ���ڸ��������գ��������ȵ�TiC��д���÷�Ӧ�Ļ�ѧ����ʽ ��
�ǽ�Al(OH)3��Na2CO3�Ļ����һͬ�����������Ƶõ�ⷨ����������ۼ�����ʯ��Na3AlF6����ͬʱ��Ӧ�в���һ����ɫ��ζ�����壬��д���йػ�ѧ����ʽ_________________��
��Na2O2��2Na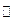 2Na2O ��2KNO3��10K
2Na2O ��2KNO3��10K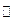 6K2O��N2
6K2O��N2
��4Al��3TiO2��3C 2Al2O3��3TiC
2Al2O3��3TiC
��2Al(OH)3��12HF��3Na2CO3��2Na3AlF6��3CO2��9H2O ��1��1��1��1��
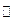 2Na2O ��2KNO3��10K
2Na2O ��2KNO3��10K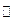 6K2O��N2
6K2O��N2��4Al��3TiO2��3C
 2Al2O3��3TiC
2Al2O3��3TiC��2Al(OH)3��12HF��3Na2CO3��2Na3AlF6��3CO2��9H2O ��1��1��1��1��
��

��ϰ��ϵ�д�
�����Ŀ

