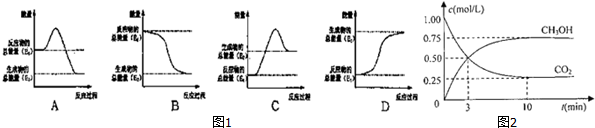
��Ŀ����
9���ɵ���A�ͻ�����B���ַ�ĩ��ɵĻ�����֪A���Ӱ뾶�ǵ�������Ԫ�������Ӱ뾶��С�ģ���һ�������¿ɰ���ͼ��ʾ��ϵ����ת����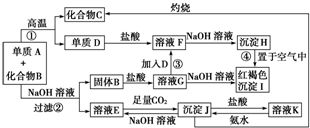
�������Ϲ�ϵ��д��
��1��A��ԭ�ӽṹʾ��ͼ

��2��B�еĻ�ѧ���������Ӽ�
��3���۲���Ӧ�����ӷ���ʽ2Fe3++Fe=3Fe2+
��4����H��I�Ļ�ѧ����ʽ4Fe��OH��2+O2+2H2O=4Fe��OH��3
��5��G�������ӵļ��鷽��ȡ����G����Һ���Թ��У������м���KSCN��Һ������Һ�������Fe3+���������Fe3+��
���� A���Ӱ뾶�ǵ�������Ԫ�������Ӱ뾶��С�ģ���AΪAl�����ɫ����IΪFe��OH��3������H���ڿ����б��I���Ƴ�HΪFe��OH��2������D�����ᷴӦF��F���������Ʒ�Ӧ������������������DΪFe��FΪFeCl2����ת����ϵ��֪��BΪFe2O3��GΪFeCl3������Al��Fe2O3��Ӧ����Fe������������CΪAl2O3����������������Ʒ�Ӧ��Ӧ�õ�EΪNaAlO2����JΪAl��OH��3��KΪAlCl3��������������ֽ��������������ݴ˽��
��� �⣺A���Ӱ뾶�ǵ�������Ԫ�������Ӱ뾶��С�ģ���AΪAl�����ɫ����IΪFe��OH��3������H���ڿ����б��I���Ƴ�HΪFe��OH��2������D�����ᷴӦF��F���������Ʒ�Ӧ������������������DΪFe��FΪFeCl2����ת����ϵ��֪��BΪFe2O3��GΪFeCl3������Al��Fe2O3��Ӧ����Fe������������CΪAl2O3����������������Ʒ�Ӧ��Ӧ�õ�EΪNaAlO2����JΪAl��OH��3��KΪAlCl3��������������ֽ�������������
��1��AΪAl��ԭ�ӽṹʾ��ͼΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��2��BΪFe2O3���������Ӽ����ʴ�Ϊ�����Ӽ���
��3���۲���Ӧ�����ӷ���ʽΪ��2Fe3++Fe=3Fe2+���ʴ�Ϊ��2Fe3++Fe=3Fe2+��
��4����H��I�Ļ�ѧ����ʽΪ��4Fe��OH��2+O2+2H2O=4Fe��OH��3 ���ʴ�Ϊ��4Fe��OH��2+O2+2H2O=4Fe��OH��3 ��
��5��GΪFeCl3������G�������ӵķ���Ϊ��ȡ����G����Һ���Թ��У������м���KSCN��Һ������Һ�������Fe3+���������Fe3+��
�ʴ�Ϊ��ȡ����G����Һ���Թ��У������м���KSCN��Һ������Һ�������Fe3+���������Fe3+��
���� ���⿼��������ƶϣ��漰Fe��AlԪ�ػ�����������ת����IΪ���ɫ������J�����ᡢ�Ӧ��Ϊ�ƶ�ͻ�ƿڣ���Ҫѧ����������Ԫ�ػ��������ʣ��Ѷ��еȣ�

| A�� | 70.7 | B�� | 70.2 | C�� | 72 | D�� | 73.3 |
| A�� | ${\;}_{48}^{108}$Cd��${\;}_{48}^{110}$Cd������48������ | |
| B�� | ${\;}_{48}^{108}$Cd��${\;}_{48}^{110}$Cd��Ϊͬλ�� | |
| C�� | ${\;}_{48}^{108}$Cd��${\;}_{48}^{110}$Cd�ĺ����������ͬ | |
| D�� | ${\;}_{48}^{108}$Cd��${\;}_{48}^{110}$Cd����60��62������ |
| A�� | �������ǻ��Ա�����Ӱ����ʹ��������ԭ�Ӷ���ĺܻ��� | |
| B�� | ���ڼ��������µ�ˮ��������Ӧ������ʹ� | |
| C�� | ����������Ӧ������һ�������� | |
| D�� | �ܷ���������Ӧ�����ʲ�һ����ȩ |
| ����\Ԫ�� | 8O | 16S | 34Se | 52Te |
| �����۵㣨�棩 | -218.4 | 113 | 450 | |
| ���ʷе㣨�棩 | -183 | 444.6 | 685 | 1390 |
| ��Ҫ���ϼ� | -2 | -2��+4��+6 | -2��+4��+6 | |
| ԭ�Ӱ뾶 | ������ | |||
| ������H2��Ӧ | ��ȼʱ���� | ���Ȼ��� | �����ѻ��� | ����ֱ�ӻ��� |
��1�������۵㷶Χ������113�桫450��
��2���ڵĻ��ϼۿ�����-2��+4��+6
��3���������н�ǿ�Ļ�ԭ�ԣ�������ԡ���ԭ�ԡ�������˰������ڿ����г��ڱ������ױ��ʣ�����ܷ����Ļ�ѧ����ʽΪ2H2Se+O2=2H2O+2Se����
| A�� | C6H5ONa+H2O+CO2��C6H5OH+NaHCO3 | |
| B�� | C6H5ONa+H2O+CO2��C6H5OH+Na2CO3 | |
| C�� | 2C6H5OH+Na2CO3��2C6H5ONa+CO2��+H2O | |
| D�� | C6H5OH+NaHCO3��C6H5ONa+H2CO3 |
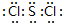 ��������ѧ������Ϊ���ۼ���
��������ѧ������Ϊ���ۼ���