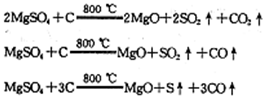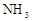��Ŀ����
�����10�֣�
��������(NOCl)���л��ϳ��е���Ҫ�Լ�������NO��Cl2��ͨ�������·�Ӧ�õ�(NO2��Cl2��Ӧ�ɵ�������)���������ȵ��۵�Ϊ��64.5 �棬�е�Ϊ��5.5 �棬������ˮ�����ֽ�Ϊ������������Ȼ��⡣
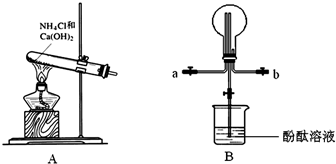
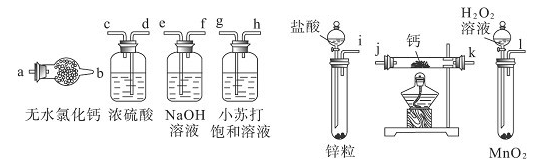
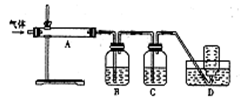
ijУ��ѧ��ȤС�鰴���������Ʊ��������ȣ�ʵ��ʱ����ͨ��Cl2����E��U�ι��ڳ�������ɫ����ʱ���ٻ���ͨ��NO����������ʾ��������
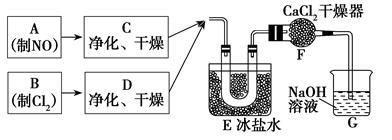
34��ʵ����������غ�Ũ�����Ʊ�Cl2�Ļ�ѧ����ʽ��______________________________��
35��װ��F��������___________________________________��
36������������ˮ��Ӧ�Ļ�ѧ����ʽ��________________________________��
37��ʵ���С���ͨ��Cl2����E��U�ι��ڳ�������ɫ����ʱ���ٽ�NO����ͨ�롱���˲�����Ŀ����______________________________________________________________��
��������(NOCl)���л��ϳ��е���Ҫ�Լ�������NO��Cl2��ͨ�������·�Ӧ�õ�(NO2��Cl2��Ӧ�ɵ�������)���������ȵ��۵�Ϊ��64.5 �棬�е�Ϊ��5.5 �棬������ˮ�����ֽ�Ϊ������������Ȼ��⡣
ijУ��ѧ��ȤС�鰴���������Ʊ��������ȣ�ʵ��ʱ����ͨ��Cl2����E��U�ι��ڳ�������ɫ����ʱ���ٻ���ͨ��NO����������ʾ��������

34��ʵ����������غ�Ũ�����Ʊ�Cl2�Ļ�ѧ����ʽ��______________________________��
35��װ��F��������___________________________________��
36������������ˮ��Ӧ�Ļ�ѧ����ʽ��________________________________��
37��ʵ���С���ͨ��Cl2����E��U�ι��ڳ�������ɫ����ʱ���ٽ�NO����ͨ�롱���˲�����Ŀ����______________________________________________________________��
34. KClO3(����)��6HCl(Ũ)��KCl��3Cl2����3H2O ��2�֣�
35. ��ֹG��ˮ��������U�ιܣ���������NOClˮ�⡣��2�֣�
36. 2NOCl��H2O �� N2O3��2HCl (��2NOCl��H2O �� NO��NO2��2HCl) ��2�֣�
37. ��ֹNO��װ���е���������ΪNO2����1�֣�����Cl2������ʹNO��ȫת��������
NO�ݳ���Ⱦ������3�֣�
���������34.��Ԫ�صĹ��з�Ӧ��35. NOCl����ˮ��Ӧ��װ��F���Է�ֹβ������װ��G��ˮ�������룻36. NOCl�е�Ԫ�ػ��ϼ�Ϊ+3�ۣ�ˮ�⻯�ϼ۲��䣬����N2O3����1:1��NO��NO2����37.װ���п�����������NO��Ӧ����NO2�������������������ȣ���Ӧ�й�����Cl2��ʹNO��ȫת��������
NO�ݳ���Ⱦ������

��ϰ��ϵ�д�
�����Ŀ