��Ŀ����
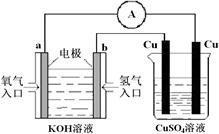
������Ƴ�����CH4��O2�ķ�Ӧ���ò��缫��KOH��Һ�й���ԭ��ء���ص��ܷ�Ӧ������CH4��O2��ȼ�գ�������˵����ȷ���ǣ� ��
��ÿ����1molCH4���������·�ṩ8mole-
�ڸ�����CH4ʧȥ���ӣ��缫��ӦʽCH4+10OH-��8e-=CO32-+7H2O
�۸�������O2��õ��ӣ��缫��ӦʽΪ O2+2H2O+4e-=4OH-
�ܵ�طŵ����ҺpH��������
��ÿ����1molCH4���������·�ṩ8mole-
�ڸ�����CH4ʧȥ���ӣ��缫��ӦʽCH4+10OH-��8e-=CO32-+7H2O
�۸�������O2��õ��ӣ��缫��ӦʽΪ O2+2H2O+4e-=4OH-
�ܵ�طŵ����ҺpH��������
| A���٢� | B���٢� | C���٢� | D���ۢ� |
A
������������������������CO2����Ӧ��ת��8�����ӣ�����ȷ��������Һ���Լ��Եģ���CO2������������������̼��أ�����ȷ�������������õ����ӣ��۲���ȷ����Ӧ�������������صģ�������Һ�ļ��Խ��ͣ�pH��С���ܲ���ȷ����ѡA��
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣�����ۺ���ǿ�����ض�ѧ������֪ʶ�Ĺ��̺�ѵ��������������ѧ�������������������ѧ��������û���֪ʶ���ʵ�����������������Ĺؼ�����ȷԭ��صĹ���ԭ����Ȼ��������ü��ɡ�

��ϰ��ϵ�д�
�����Ŀ
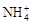 ��2e����Mn2O3��2NH3��H2O��
��2e����Mn2O3��2NH3��H2O��
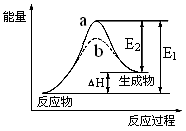
 CH3OH(g)����H ����90.8 kJ��mol��1
CH3OH(g)����H ����90.8 kJ��mol��1