��Ŀ����
��2011?ʯ��ɽ��һģ��ij��ɫ��Һ�к��У���Na+����Ba2+����Cl-����Br-����SO2-3����SO2-4-�����е�һ�ֻ��֣����ν�������ʵ�飬��ÿ�������Լ����������۲쵽���������£�
|
��������������ҺpH����7��˵����Һ�Լ��ԣ��жϴ��ڵ����ӣ�
����Һ�еμ���ˮ���ټ���CCl4������CCl4��ʳȺ�ɫ��������������������嵥�ʷ�����
���������������ɫ�����ǵ������ᱵ�������ɵ�ԭ��
�����������ж��������ӵ����ʣ��ۺ�������������Ȼ���������ԭ��
����Һ�еμ���ˮ���ټ���CCl4������CCl4��ʳȺ�ɫ��������������������嵥�ʷ�����
���������������ɫ�����ǵ������ᱵ�������ɵ�ԭ��
�����������ж��������ӵ����ʣ��ۺ�������������Ȼ���������ԭ��
����⣺����ҺpH����7��˵����Һ�Լ��ԣ�����������ʿ�֪��ֻ�������������ˮ���Լ��ԣ�������Һ��һ�����Т�SO32-��
������Һ�еμ���ˮ���ټ���CCl4������CCl4��ʳȺ�ɫ��˵����Һ�к��������ӣ���ͨ�����������Ϊ�嵥�ʣ�������Һ��һ�����Т�Br-��
��ȡ�ڵ��ϲ���Һ������Ba��NO3��2��Һ��ϡHNO3���а�ɫ�������������������ᱵ������ԭ��Һ������������ӱ��������������Ϊ����������������ᱵ����������ԭ��Һ����������Ӳ���ȷ����
�ܽ��۹��ˣ�����Һ�м���AgNO3��Һ��ϡHNO3���а�ɫ�������ɣ�˵�����Ȼ������������ڢڲ�������������ӣ�����ԭ��Һ�������Ӳ���ȷ����
��������ԭ��Һ��һ����������������Ӻ������ӣ�����ԭ��Һ����ɫ��Һ������һ��������Ba2+�����ݵ������Һ�ʵ����ԣ������������ӱ����������ӣ����һ������Na+������
A���϶����е������Ǣ٢ܢݣ���A��ȷ��
B���϶�û�е������Ǣڣ���B����
C�����ܺ��е������Ǣۢޣ���C����
D������ȷ���������Ǣۢޣ���D����
��ѡA��
������Һ�еμ���ˮ���ټ���CCl4������CCl4��ʳȺ�ɫ��˵����Һ�к��������ӣ���ͨ�����������Ϊ�嵥�ʣ�������Һ��һ�����Т�Br-��
��ȡ�ڵ��ϲ���Һ������Ba��NO3��2��Һ��ϡHNO3���а�ɫ�������������������ᱵ������ԭ��Һ������������ӱ��������������Ϊ����������������ᱵ����������ԭ��Һ����������Ӳ���ȷ����
�ܽ��۹��ˣ�����Һ�м���AgNO3��Һ��ϡHNO3���а�ɫ�������ɣ�˵�����Ȼ������������ڢڲ�������������ӣ�����ԭ��Һ�������Ӳ���ȷ����
��������ԭ��Һ��һ����������������Ӻ������ӣ�����ԭ��Һ����ɫ��Һ������һ��������Ba2+�����ݵ������Һ�ʵ����ԣ������������ӱ����������ӣ����һ������Na+������
A���϶����е������Ǣ٢ܢݣ���A��ȷ��
B���϶�û�е������Ǣڣ���B����
C�����ܺ��е������Ǣۢޣ���C����
D������ȷ���������Ǣۢޣ���D����
��ѡA��
���������⿼���˳������Ӽ���ķ������ؼ������ӵĹ����жϺ������Ӧ�ã��Լ������Լ��Բ��������Ӱ��������ã�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ

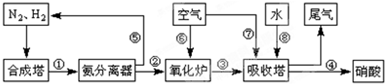
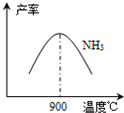 ��3��N2��H2���������������´�145��Ϳ�ʼ��Ӧ����ͬ�¶���NH3������ͼ��ʾ���¶ȸ���900��ʱ��NH3�����½���ԭ��
��3��N2��H2���������������´�145��Ϳ�ʼ��Ӧ����ͬ�¶���NH3������ͼ��ʾ���¶ȸ���900��ʱ��NH3�����½���ԭ��