��Ŀ����
��32.64 gͭ��140 mLһ��Ũ�ȵ����ᷴӦ��ͭ��ȫ�ܽ������NO��NO2��������ڱ�״���µ����Ϊ11.2 L��������������⣺��1��NO�����Ϊ___________L��NO2�����Ϊ___________L��
��2��������������ȫ���ͷź�����Һ�м���V mL a mol��L-1��NaOH��Һ��ǡ��ʹ��Һ�е�Cu2+ȫ��ת���ɳ�������ԭ�������ʵ���Ũ��Ϊ___________ mol��L-1��
��3����ʹͭ�����ᷴӦ���ɵ�������NaOH��Һ��ȫ��ת��ΪNaNO3��������Ҫ30%��˫��ˮ___________g��
��1��5.8 5.4
(2)![]() (3)57.7
(3)57.7
������(1)������NO�����Ϊx��NO2�����Ϊy,�ɽ������̣�x+y=11.2 L�����ɵ�ʧ�����غ�֪��Cuʧȥ�ĵ���������NO��NO2�õ��ĵ��������ֿɽ������̣�
![]() ��1,����������ɽ�ã�x=5.8 L,y=5.4 L��
��1,����������ɽ�ã�x=5.8 L,y=5.4 L��
��2������ԭ�����е�Ԫ���غ㣺N������NO��NO2������壬NaNO3��ʣ���HNO3��ʽ��������Һ�С�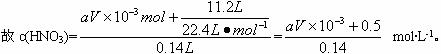
��3��H2O2����������NO��NO2�����������ԭ���������ݵ�ʧ�����غ�ɵã�
![]() ����(-1)-(-2)�ݡ�2=
����(-1)-(-2)�ݡ�2=![]() ����(+5)-(+2)��+
����(+5)-(+2)��+![]() ���ۣ�+5��-��+4���ݣ����m(H2O2)=57.7 g��
���ۣ�+5��-��+4���ݣ����m(H2O2)=57.7 g��

��ϰ��ϵ�д�
 �߲������Ӧ��һ��ͨϵ�д�
�߲������Ӧ��һ��ͨϵ�д�
�����Ŀ