��Ŀ����
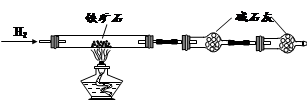
��֪Fe2O3��H2��Ӧ�����¶Ȳ�ͬ��������Fe3O4���ɡ�ij��ѧ��ȤС������H2��ԭFe2O3��ʵ���У��ô����������ɵĺ�ɫ��ĩX��Ϊ̽��X����ɣ����ǽ���������ʵ�飺
(1)��ͬѧ��Ϊ��ɫ��ĩX�ܱ��������������X����������ͬѧ��ͬ�����Ľ��ۣ�ԭ����___________________________��
(2)��ͬѧ�Ƚ�������ɫ��ĩX����װ����������ͭ��Һ���ձ��У������岿���ܽ⣬�м�������ɫ�������������ˣ�Ȼ���������м������ᣬ�ٵμӼ���KSCN��Һ����Һ���ֺ�ɫ��ͨ�������������ͬѧ�ó�X�������Fe��Fe3O4��
�ٵμ�KSCN��Һ��Ŀ����_____________________��
��������ĩXֱ�Ӽ��������У��ټ�KSCN��Һ����Һ�����ֺ�ɫ�����ֺ�ɫ��ԭ����(�����ӷ���ʽ��ʾ) _____________��
(3)��ͬѧ��ʵ�鷽����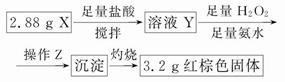
�ٲ���Z��__________________��
��ͨ���������ݣ��ó�2.88 g��ɫ��ĩX�и��ɷֵ����ʵ���Ϊ____________________________________��
(1)Fe3O4Ҳ�ܱ������������ʲ����ɴ�ȷ��X����������
(2)�ټ����Ƿ����Fe3����ȷ��Fe3O4�Ĵ���
��Fe��2Fe3��=3Fe2��
(3)�ٹ��ˡ�ϴ��
��n(Fe3O4)��0.01 mol��n(Fe)��0.01 mol
����

��15�֣�Fe2O3�׳��������죬������������ɫ������ҵ��ú��ʯ����Ҫ�ɷ֣�SiO2 49.5%��Fe2O3 20.6%��Al2O318.9%���Լ�MgO��FeO���������ʣ��Ʊ����о����������Ӧ�á�
��һ���Ʊ���������
1��Ԥ��������ú��ʯ���飬��350���±���2Сʱ��
2�������ܽ⣺��Ԥ�������ú��ʯ������������Ϊ15%������������Һ�У����ˡ�����Һ�м����H2O2��
3������pH����������Һ�м�1mol/LNaOH��Һ������Һ��pH���ٹ��ˣ��õ�������
4����Ʒ����������������ˮϴ����ɡ����ա���ĥ����ɸ�ò�Ʒ��
��֪����������������������ʽ����ʱ��Һ��pH���±�
| ������ | Fe(OH)3 | Al(OH)3 | Fe(OH)2 | Mg(OH)2 |
| ��ʼ���� | 1.3 | 3.3 | 7.5 | 10.3 |
| ��ȫ���� | 2.8 | 5.2 | 9.7 | 12.2 |
��1��Ԥ����ʱ����ú��ʯ����2Сʱ��Ŀ���� ��
��2���������������H2SO4�������� ��
��ʵ�����н��иò���ʱ���õ����������� �� ��
��3����NaOH��Һ����pH����ѷ�Χ�� �����ӵڶ��ι��˵���Һ�л�ȡ�ϴ���������þ���壬Ӧ����IJ����� ��ϴ�Ӻ��T�á�
��4����Ʒ����ʱ��������ˮϴ�IJ����� ��
���������������ۣ�
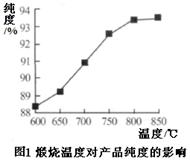
��5����Ʒ����ʱ�������¶ȶԲ�Ʒ�Ĵ����кܴ�Ӱ�졣��֪�¶ȶԲ��﴿�ȵ�Ӱ����ͼ1��ʾ��������ʱ�¶���ÿ����� �档
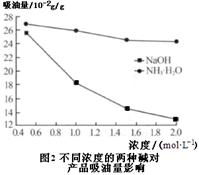
��6���������Ƿ�ӳ��������������ʵ���Ҫָ�ꡣ��������˵���������������ϴ������������л��������ٳ���Ӱ����������ͬŨ�ȵ����ּ���Һ�Բ���������Ӱ����ͼ2��ʾ���������������ڵ�����ҺpHʱ��ѡ��NaOH��Һ����ѡ�ð�ˮ��ԭ���� ��
Fe2����I�������ֳ����Ļ�ԭ�����ӡ�
��1����FeSO4��Һ�еμ���ˮ����Һ��dz��ɫ��ɻ�ɫ����Ӧ�����ӷ���ʽΪ ����KI��Һ�еμ���ˮ����Һ����ɫ��ɻ�ɫ����Ӧ�����ӷ���ʽ�� ��
��2������FeSO4��Һ��KI��Һ����ˮΪ�Լ���֤I���Ļ�ԭ��ǿ��Fe2�������ʵ�鷽�����������ʵ�鲽�衢Ԥ������ͽ��ۡ�������ѡ�Լ���3 mol��L��1 H2SO4��0.01 mol��L��1 KMnO4��20% KSCN��3%H2O2��������Һ����ɫʯ����Һ��
| ʵ�鲽�� | Ԥ����������� |
| ����1��ȡ2mLFeSO4��Һ��2mLKI��Һ������Թ��У��ٵμ�1��2����ˮ�� | �� |
| ����2��____________________________________ ____________________________________�� | |
��3�����ã�2���ṩ���Լ�֤���������Ļ����������ԣ�2�ۣ�ʵ������������ǣ�ȡ������Ʒ����ˮ�� ��
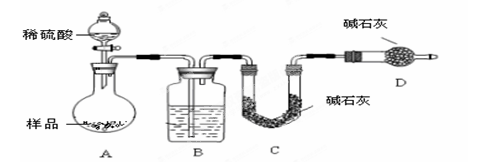
����������FeC2O4�������������Լ�����Ӱ���Լ����͵�ز�����������﮵����������������ڸ�������ʱ�����ܹ��ֽ�,��ȤС��Բ��������ķֽ���������ʵ���̽��������֪��CO�����Ȼ���[PdC12]��Һ��Ӧ���ɺ�ɫ���ٷۡ���
��1�������������ֽ�������������ͨ������ʯ��ˮ���Ȼ�����Һ���۲쵽����ʯ��ˮ����ǣ��Ȼ�����Һ���к�ɫ�������ɡ�˵������������� �����ѧʽ��
��2��̽�����������ֽ�õ��ĺ�ɫ�����������Ԫ�صĴ�����ʽ��
���������⡿
���������ֽ��õ��ĺ�ɫ������ʲô��
��������衿
����1�� ������2��FeO������3��FeO��Fe�Ļ���
��ʵ�鷽����
��ѡ�Լ������ᡢ��ˮ��CuSO4��Һ��KSCN��Һ������ˮ��
| ʵ�鲽�� | ʵ������ | ʵ����� |
| ����1�����Թ��м��������������ټ������� ������� | ����Һ��ɫ���Ըı䣬 ���к�ɫ�������ɡ� | ��Fe���ڡ� |
| ����2��������1�еõ�����Һ���ˣ���������ˮ������ϴ����ϴ��Һ����ɫ�� | | |
| ����3��ȡ����2�õ��������������Թ��У��μӹ������ᣬ���ú�ȡ�ϲ���Һ�� �� | �� | ��FeO���ڡ� |
����˼������
����ȤС�����۷�����Ϊ����������ֱ�ӷֽ����ù������Ӧ����FeO�������չ�������л�����Fe����Ϊ ��д��ѧ����ʽ����
��3������ʵ��̽���ͷ�˼��д�����������ڸ�������ʱ����ֱ�ӷֽ�Ļ�ѧ����ʽ ��
I������ռ������Ҫ�Ļ���ԭ�ϡ�
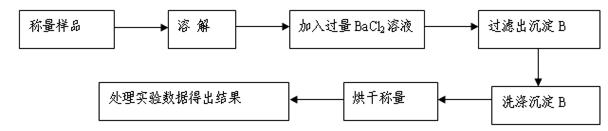
��1��������ͼ��ʾװ�ÿɼ��֤��������̼���ռ���Һ�����˷�Ӧ����A��B���ӣ���ֹˮ�У�����ͷ�ι��е�Һ�強����ƿ����ʱ��ʵ��������___________________��
�������������䣬��A��C���ӣ��ɹ۲쵽��������__________________________��
��2����NaOH��Һ��ͨ��һ����CO2���ᾧ��õ���ɫ���壬�ð�ɫ�������ɿ����ǣ�
A��NaOH��Na2CO3��B����������������C������������������D����������������
��3�����ʵ��ȷ����2���а�ɫ�����д���A���е������ӣ�
| ʵ����� | ʵ������ | ���� |
| ��ȡ������ɫ�������Թ��У�������ˮ�ܽ⣬�ټ�����BaCl2��Һ | | |
| �� | | |
II����ѧ��ȤС���ijƷ�������е�Ħ�����ɷּ��京����������̽����
������ϣ�������Ħ������̼��ƣ�����������ɣ������������ɷ���������ʱ�����������
������Ʒ��̼��ƵĶ����ⶨ��������ͼ��ʾװ��(ͼ�мг�������ȥ)����ʵ�飬��ַ�Ӧ
�ⶨC�����ɵ�BaCO3������������ȷ��̼��Ƶ�����������

����ʵ����̻ش��������⣺
��1��ʵ����������������ͨ������������ó��˿ɽ���B��C�еķ�Ӧ���⣬���У� ��
��2��C�з�Ӧ����BaCO3�����ӷ���ʽ�� ��
��3�����и����ʩ�У�������߲ⶨȷ�ȵ��ǣ� ��
A���ڼ�������֮ǰ��Ӧ�ž�װ���ڵ�CO2����
B���μ�����˹���
C����A��B֮������ʢ��Ũ�����ϴ��װ��
D����B��C֮������ʢ�б���̼��������Һ��ϴ��װ��
��4��ʵ����ȷ��ȡ8.00 g��Ʒ���ݣ��������βⶨ�����BaCO3ƽ������Ϊ3.94 g.����Ʒ��̼��Ƶ���������Ϊ________��
��5��������Ϊ���زⶨC�����ɵ�BaCO3������ֻҪ�ⶨװ��C������CO2ǰ��������һ������ȷ��̼��Ƶ�����������ʵ��֤�����˷����ⶨ�Ľ������ƫ�ߣ�ԭ����_________________________________��
��6��װ����U�ι�D�еļ�ʯ�ҵ�������_____________________________��