��Ŀ����
ʳ��Ϊ��������ƴ����֣����ұ��涨����ʳ�����Ậ�����õ���3.5g/100mL��
��1��ij�о�С�����õζ��������ⶨijƷ��ʳ���д���ĺ���������˵����ȷ����
a����NaOH��Һ�ζ�ʱ��Ӧ�����ӷ���ʽΪ��H++OH-�TH2O
b��ʳ��������ϡ��һ���������ٽ��еζ�
c����NaOH��Һ�ζ�ʳ�ף���ʹ�÷�̪�������ָʾ��
d������ø�Ʒ��ʳ�����ʵ���Ũ��Ϊ0.75mol/L�����ʳ��������Ϊ4.5g/100mL
��2���о�С���ͬѧ��ϸ�۲��˸�Ʒ��ʳ�ı�ǩ���������л����б���������ΪʳƷ���Ӽ������������Ϸ���֤������ʳƷ���Ӽ�����������C6H5COONa�����ᷢ�����ӻ�����Ӧ���������һ���¶��µĴ����뱽�����
a��pH b������� c�����볣�� d���ܽ��
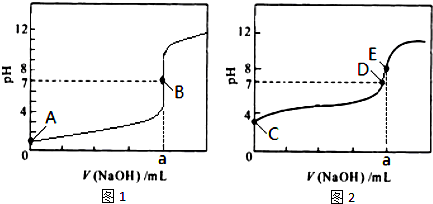
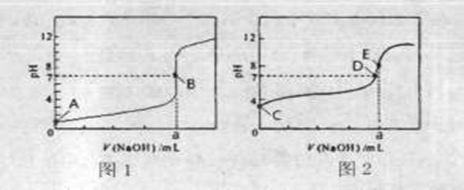
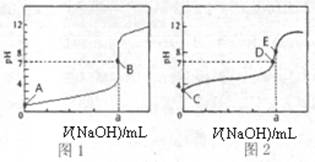
��3�������£���0.1000mol?L-1 NaOH��Һ�ֱ�ζ�20.00mL 0.1000mol?L-1 HCl��Һ��20.00mL 0.1000mol?L-1 CH3COOH��Һ���õ�2���ζ����ߣ���ͼ��ʾ��
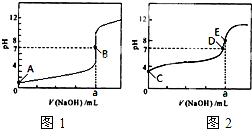
�ٵζ�������Һ��������
��E��pH��8��ԭ�������ӷ���ʽ��ʾ
��4���������Բⶨһδ֪Ũ�ȵ�CH3COOH��Һ�ĵ���ȣ�Ӧ����ʵ���������Լ�����ֽ����
a����⣬NaOH��Һ b������Na2CO3��Һ
c��������Ӧ��ʯ����Һ d���к͵ζ���pH��ֽ
��5��NaOH��Һ�ζ�CH3COOH��Һ��ѡ��
��1��ij�о�С�����õζ��������ⶨijƷ��ʳ���д���ĺ���������˵����ȷ����
bd
bd
a����NaOH��Һ�ζ�ʱ��Ӧ�����ӷ���ʽΪ��H++OH-�TH2O
b��ʳ��������ϡ��һ���������ٽ��еζ�
c����NaOH��Һ�ζ�ʳ�ף���ʹ�÷�̪�������ָʾ��
d������ø�Ʒ��ʳ�����ʵ���Ũ��Ϊ0.75mol/L�����ʳ��������Ϊ4.5g/100mL
��2���о�С���ͬѧ��ϸ�۲��˸�Ʒ��ʳ�ı�ǩ���������л����б���������ΪʳƷ���Ӽ������������Ϸ���֤������ʳƷ���Ӽ�����������C6H5COONa�����ᷢ�����ӻ�����Ӧ���������һ���¶��µĴ����뱽�����
c
c
����д��ţ���a��pH b������� c�����볣�� d���ܽ��
��3�������£���0.1000mol?L-1 NaOH��Һ�ֱ�ζ�20.00mL 0.1000mol?L-1 HCl��Һ��20.00mL 0.1000mol?L-1 CH3COOH��Һ���õ�2���ζ����ߣ���ͼ��ʾ��

�ٵζ�������Һ��������
ͼ2
ͼ2
���ͼ1����ͼ2�������ζ�������a=20
20
mL����E��pH��8��ԭ�������ӷ���ʽ��ʾ
CH3COO-+H2O?CH3COOH+OH-
CH3COO-+H2O?CH3COOH+OH-
����4���������Բⶨһδ֪Ũ�ȵ�CH3COOH��Һ�ĵ���ȣ�Ӧ����ʵ���������Լ�����ֽ����
d
d
��a����⣬NaOH��Һ b������Na2CO3��Һ
c��������Ӧ��ʯ����Һ d���к͵ζ���pH��ֽ
��5��NaOH��Һ�ζ�CH3COOH��Һ��ѡ��
��̪
��̪
��ָʾ�����յ������Ϊ����ɫ��ۺ�ɫ���Ұ�����ڲ��ָ�ԭɫ
����ɫ��ۺ�ɫ���Ұ�����ڲ��ָ�ԭɫ
����װ����Һ����ƿδ�ô�װҺ��ϴ�����Ĵ���ĺ�����Ӱ��
��Ӱ��
���ƫ�ߡ�����ƫ�͡�����Ӱ�족����������1��a�����ݴ������������Ӧ��д�ɷ���ʽ�жϣ�
b��ϡ�ͺ���Լ�С�ζ���
c�����ȵı�ʾ��ΧΪ3.1-4.4�����ܹ�ʹ�ü�����ָʾ����
d������ʳ�����ʵ���Ũ��Ϊ0.75mol/L������������������
��2�����ݵ��볣���������������ǿ��������
��3���ٸ��ݼ�������������Һ�����Ϊ0ʱ��Һ��pH�жϣ�����������Һ��ͼʾ�ж�a����ֵ��
��E�������˴�������Һ�����ݲ�������ӵ�ˮ�������Һ��ʾ����ԭ��
��4���ⶨ����ĵ���ȣ���Ҫ�����к͵ζ���ͨ���ⶨ��Һ��pH�жϣ�
��5���������Ƶζ�������Һ����Ҫ��Ҫ��̪��ָʾ�������ݵζ�����ǰ��Һ��ʾ��ɫ���ζ���������Һ��ʾ���ԣ���̪��ʾ��ɫ�жϵζ��յ㣻ʢװ����Һ����ƿ�����ô���Һ��ϴ��
b��ϡ�ͺ���Լ�С�ζ���
c�����ȵı�ʾ��ΧΪ3.1-4.4�����ܹ�ʹ�ü�����ָʾ����
d������ʳ�����ʵ���Ũ��Ϊ0.75mol/L������������������
��2�����ݵ��볣���������������ǿ��������
��3���ٸ��ݼ�������������Һ�����Ϊ0ʱ��Һ��pH�жϣ�����������Һ��ͼʾ�ж�a����ֵ��
��E�������˴�������Һ�����ݲ�������ӵ�ˮ�������Һ��ʾ����ԭ��
��4���ⶨ����ĵ���ȣ���Ҫ�����к͵ζ���ͨ���ⶨ��Һ��pH�жϣ�
��5���������Ƶζ�������Һ����Ҫ��Ҫ��̪��ָʾ�������ݵζ�����ǰ��Һ��ʾ��ɫ���ζ���������Һ��ʾ���ԣ���̪��ʾ��ɫ�жϵζ��յ㣻ʢװ����Һ����ƿ�����ô���Һ��ϴ��
����⣺��1��a����NaOH��Һ�ζ�ʱ��������������ʣ�Ӧ�����ɷ���ʽ����Ӧ�����ӷ���ʽΪ��CH3COOH+OH-�TH2O+CH3COO-����a����
b�����˼�С�ζ����ζ�ǰ��ʳ��������ϡ��һ���������ٽ��еζ�����b��ȷ��
c����NaOH��Һ�ζ�ʳ�ף����ڼ��ȵı�ɫ��ΧΪ3.1-4.4�����ܹ�ʹ�ü�����Ϊָʾ������ʹ�÷�̪��ָʾ������c����
d������ø�Ʒ��ʳ�����ʵ���Ũ��Ϊ0.75mol/L�������Ħ������Ϊ60g/mol��100mL����0.075mol���ᣬ���������Ϊ4.5g�����ʳ��������Ϊ4.5g/100mL����d��ȷ��
��ѡ��bd��
��2������֤������ʳƷ���Ӽ�����������C6H5COONa�����ᷢ�����ӻ�����Ӧ����Ҫ֤�����������С�ڱ���������ԣ�����ĵ��볣���������������ǿ����������Ҫ������һ���¶��µĴ����뱽����ĵ��볣����
��ѡ��c��
��3���ٳ����£���0.1000mol?L-1 NaOH��Һ�ֱ�ζ�20.00mL 0.1000mol?L-1 HCl��Һ��20.00mL 0.1000mol?L-1 CH3COOH��Һ����������������Һǰ����������ǿ����ʣ���ȫ���룬��Һ��pHΪ1������������������ʣ�������Һ��pHһ������1������ͼ1��������Һ�ζ����ߣ�ͼ2�Ǵ�����Һ�ĵζ����ߣ�20.00mL 0.1000mol?L-1 HCl��Һ�У���Ҫ����20mL 0.1000mol?L-1 NaOH��Һ����Һǡ�÷�Ӧ����Һ��pH=7������a=20��
�ʴ�Ϊ��ͼ2��20��
���ڼ���20mL����������Һ�������������Ʒ�Ӧ�����˴����ƣ���������ӷ���ˮ�⣬��Һ��ʾ���ԣ���Ӧ�����ӷ���ʽΪ��CH3COO-+H2O?CH3COOH+OH-��
�ʴ�Ϊ��CH3COO-+H2O?CH3COOH+OH-��
��4�������Բⶨһδ֪Ũ�ȵ�CH3COOH��Һ�ĵ���ȣ���Ҫ��������к͵ζ��ⶨ��ͨ���ⶨ�ζ���������Һ��pH�仯����ʹ�õ�pH��ֽ��
��ѡ��d��
��5����̪�ı�ɫ��ΧΪ8-10��NaOH��Һ�ζ�CH3COOH��Һ������ѡ�÷�̪��ָʾ�����ζ�����ʱ��Һ����ɫ��ɺ�ɫ���ζ�ʱ��ƿ���ܹ��ô���Һ��ϴ������װ����Һ����ƿδ�ô�װҺ��ϴ����Ӱ��ζ������
�ʴ�Ϊ����̪�� ����ɫ��ۺ�ɫ���Ұ�����ڲ��ָ�ԭɫ����Ӱ�죮
b�����˼�С�ζ����ζ�ǰ��ʳ��������ϡ��һ���������ٽ��еζ�����b��ȷ��
c����NaOH��Һ�ζ�ʳ�ף����ڼ��ȵı�ɫ��ΧΪ3.1-4.4�����ܹ�ʹ�ü�����Ϊָʾ������ʹ�÷�̪��ָʾ������c����
d������ø�Ʒ��ʳ�����ʵ���Ũ��Ϊ0.75mol/L�������Ħ������Ϊ60g/mol��100mL����0.075mol���ᣬ���������Ϊ4.5g�����ʳ��������Ϊ4.5g/100mL����d��ȷ��
��ѡ��bd��
��2������֤������ʳƷ���Ӽ�����������C6H5COONa�����ᷢ�����ӻ�����Ӧ����Ҫ֤�����������С�ڱ���������ԣ�����ĵ��볣���������������ǿ����������Ҫ������һ���¶��µĴ����뱽����ĵ��볣����
��ѡ��c��
��3���ٳ����£���0.1000mol?L-1 NaOH��Һ�ֱ�ζ�20.00mL 0.1000mol?L-1 HCl��Һ��20.00mL 0.1000mol?L-1 CH3COOH��Һ����������������Һǰ����������ǿ����ʣ���ȫ���룬��Һ��pHΪ1������������������ʣ�������Һ��pHһ������1������ͼ1��������Һ�ζ����ߣ�ͼ2�Ǵ�����Һ�ĵζ����ߣ�20.00mL 0.1000mol?L-1 HCl��Һ�У���Ҫ����20mL 0.1000mol?L-1 NaOH��Һ����Һǡ�÷�Ӧ����Һ��pH=7������a=20��
�ʴ�Ϊ��ͼ2��20��
���ڼ���20mL����������Һ�������������Ʒ�Ӧ�����˴����ƣ���������ӷ���ˮ�⣬��Һ��ʾ���ԣ���Ӧ�����ӷ���ʽΪ��CH3COO-+H2O?CH3COOH+OH-��
�ʴ�Ϊ��CH3COO-+H2O?CH3COOH+OH-��
��4�������Բⶨһδ֪Ũ�ȵ�CH3COOH��Һ�ĵ���ȣ���Ҫ��������к͵ζ��ⶨ��ͨ���ⶨ�ζ���������Һ��pH�仯����ʹ�õ�pH��ֽ��
��ѡ��d��
��5����̪�ı�ɫ��ΧΪ8-10��NaOH��Һ�ζ�CH3COOH��Һ������ѡ�÷�̪��ָʾ�����ζ�����ʱ��Һ����ɫ��ɺ�ɫ���ζ�ʱ��ƿ���ܹ��ô���Һ��ϴ������װ����Һ����ƿδ�ô�װҺ��ϴ����Ӱ��ζ������
�ʴ�Ϊ����̪�� ����ɫ��ۺ�ɫ���Ұ�����ڲ��ָ�ԭɫ����Ӱ�죮
���������⿼�����к͵ζ����ѶȲ����漰�˵ζ�������ָʾ����ѡ�ζ������жϡ��ζ���������֪ʶ����ֿ�����ѧ���ķ������������������Ӧ��֪ʶ��������

��ϰ��ϵ�д�
 �����Ļ������������������ϵ�д�
�����Ļ������������������ϵ�д�
�����Ŀ