��Ŀ����
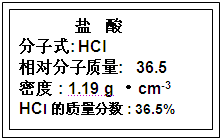
��ͼΪʵ����ij�Լ�ƿ��ǩ�ϵ��й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺
| ����������Һ ����ʽ��NaOH ��Է���������40 �ܶȣ�1.2g?cm-3 ����������20% |
��2������Ҫ���Ƹ�Ũ�ȵ�NaOH��Һ100ml�������______g�����������ƣ���Һ���Ƶ�����Ļ����������£�
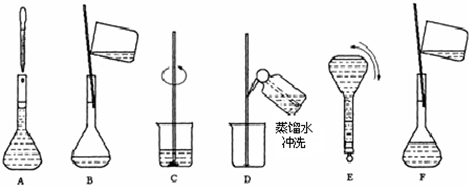
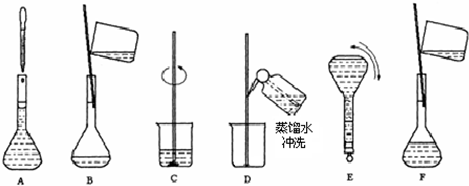
��3��������ʵ�鲽��A��F��ʵ������Ⱥ��������______��
��4������ʵ�鲽��A��B��E��F���õ�����������Ϊ______��
��5�����в�����NaOH��Һ�����ʵ���Ũ���к�Ӱ�죨�ƫ�ߡ�����ƫ�͡�����Ӱ�족����
��ҡ�Ⱥ���Һ����ڿ̶����ټ�ˮ______��
������ƿ��ԭ����������ˮ______��
�۶���ʱ���ӹ۲�Һ��______��
�⣺��1����NaOH��Һ�����ʵ���Ũ��Ϊ��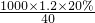 mol/L=6mol/L���ʴ�Ϊ��6��
mol/L=6mol/L���ʴ�Ϊ��6��
��2������6mol/L��NaOH��Һ100ml��Ҫ�������Ƶ�����Ϊ��0.1L��6mol/L��40g/mol=24.0g���ʴ�Ϊ��24.0��
��3����Һ���Ʋ��裺������������ܽ����ȴ��ת�ơ�ϴ�ӡ����ݡ�ҡ�ȣ�����Һ����ʵ������Ⱥ��������Ϊ��CBDFAE���ʴ�Ϊ��CBDFAE��
��4����ͼ��֪������ʵ�鲽��A��B��E��F���õ�����������Ϊ100ml����ƿ���ʴ�Ϊ��100ml����ƿ��
��5����ҡ�Ⱥ���Һ����ڿ̶��ߣ�������Һ������ƿ����ƿ��֮�䣬�ټ�ˮ���̶��ߣ�����������Һ�����ƫ��������Һ��Ũ��ƫ�ͣ�
�ʴ�Ϊ��ƫ�ͣ�
����Һ�������ˮ���ݣ�����ƿ��ԭ����������ˮ����������Һ��Ӱ�죬�ʴ�Ϊ����Ӱ�죻
�۶���ʱ���ӹ۲�Һ�棬����������Һ�����ƫС��������Һ��Ũ��ƫ�ߣ��ʴ�Ϊ��ƫ�ߣ�
��������1������c=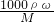 �����NaOH��Һ�����ʵ���Ũ�ȣ�
�����NaOH��Һ�����ʵ���Ũ�ȣ�
��2������m=nM=cVM�������Ƹ�Ũ�ȵ�NaOH��Һ100ml��Ҫ�������Ƶ�������
��3��������Һ���ƵIJ����������������Һ���Ʋ��裺������������ܽ����ȴ��ת�ơ�ϴ�ӡ����ݡ�ҡ�ȣ�
��4����ͼ��֪������ʵ�鲽��A��B��E��F���õ�����������Ϊ100ml����ƿ��
��5�������������������ʵ�������Һ�����Ӱ�죬����c= �ж϶�������ҺŨ�ȵ�Ӱ�죮
�ж϶�������ҺŨ�ȵ�Ӱ�죮
���������⿼�����ʵ���Ũ�ȼ��㡢�ƶ����ʵ���Ũ����Һ�����ƣ��ѶȲ���ע�����c= ������Һ��������������
������Һ��������������
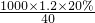 mol/L=6mol/L���ʴ�Ϊ��6��
mol/L=6mol/L���ʴ�Ϊ��6����2������6mol/L��NaOH��Һ100ml��Ҫ�������Ƶ�����Ϊ��0.1L��6mol/L��40g/mol=24.0g���ʴ�Ϊ��24.0��
��3����Һ���Ʋ��裺������������ܽ����ȴ��ת�ơ�ϴ�ӡ����ݡ�ҡ�ȣ�����Һ����ʵ������Ⱥ��������Ϊ��CBDFAE���ʴ�Ϊ��CBDFAE��
��4����ͼ��֪������ʵ�鲽��A��B��E��F���õ�����������Ϊ100ml����ƿ���ʴ�Ϊ��100ml����ƿ��
��5����ҡ�Ⱥ���Һ����ڿ̶��ߣ�������Һ������ƿ����ƿ��֮�䣬�ټ�ˮ���̶��ߣ�����������Һ�����ƫ��������Һ��Ũ��ƫ�ͣ�
�ʴ�Ϊ��ƫ�ͣ�
����Һ�������ˮ���ݣ�����ƿ��ԭ����������ˮ����������Һ��Ӱ�죬�ʴ�Ϊ����Ӱ�죻
�۶���ʱ���ӹ۲�Һ�棬����������Һ�����ƫС��������Һ��Ũ��ƫ�ߣ��ʴ�Ϊ��ƫ�ߣ�
��������1������c=
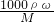 �����NaOH��Һ�����ʵ���Ũ�ȣ�
�����NaOH��Һ�����ʵ���Ũ�ȣ���2������m=nM=cVM�������Ƹ�Ũ�ȵ�NaOH��Һ100ml��Ҫ�������Ƶ�������
��3��������Һ���ƵIJ����������������Һ���Ʋ��裺������������ܽ����ȴ��ת�ơ�ϴ�ӡ����ݡ�ҡ�ȣ�
��4����ͼ��֪������ʵ�鲽��A��B��E��F���õ�����������Ϊ100ml����ƿ��
��5�������������������ʵ�������Һ�����Ӱ�죬����c=
 �ж϶�������ҺŨ�ȵ�Ӱ�죮
�ж϶�������ҺŨ�ȵ�Ӱ�죮���������⿼�����ʵ���Ũ�ȼ��㡢�ƶ����ʵ���Ũ����Һ�����ƣ��ѶȲ���ע�����c=
 ������Һ��������������
������Һ�������������� 
��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
��ͼΪʵ����ij�Լ�ƿ��ǩ�ϵ��й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺
| ����������Һ ����ʽ��NaOH ��Է���������40 �ܶȣ�1.2g?cm-3 ����������20%��1����NaOH��Һ�����ʵ���Ũ��Ϊ 6 6 mol/L����2������Ҫ���Ƹ�Ũ�ȵ�NaOH��Һ100ml������� 24.0 24.0 g�����������ƣ���Һ���Ƶ�����Ļ����������£� ��3��������ʵ�鲽��A��F��ʵ������Ⱥ�������� CBDFAE CBDFAE ����4������ʵ�鲽��A��B��E��F���õ�����������Ϊ 100ml����ƿ 100ml����ƿ ����5�����в�����NaOH��Һ�����ʵ���Ũ���к�Ӱ�죨�ƫ�ߡ�����ƫ�͡�����Ӱ�족���� ��ҡ�Ⱥ���Һ����ڿ̶����ټ�ˮ ƫ�� ƫ�� ��������ƿ��ԭ����������ˮ ��Ӱ�� ��Ӱ�� ���۶���ʱ���ӹ۲�Һ�� ƫ�� ƫ�� ��
��ͼΪʵ����ij�Լ�ƿ��ǩ�ϵ��й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺
��2������Ҫ���Ƹ�Ũ�ȵ�NaOH��Һ100ml������� g�����������ƣ���Һ���Ƶ�����Ļ����������£� 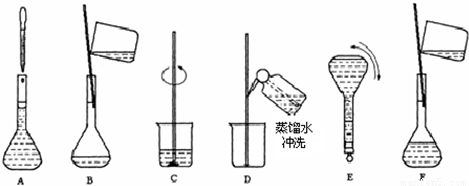 ��3��������ʵ�鲽��A��F��ʵ������Ⱥ�������� �� ��4������ʵ�鲽��A��B��E��F���õ�����������Ϊ �� ��5�����в�����NaOH��Һ�����ʵ���Ũ���к�Ӱ�죨�ƫ�ߡ�����ƫ�͡�����Ӱ�족���� ��ҡ�Ⱥ���Һ����ڿ̶����ټ�ˮ �� ������ƿ��ԭ����������ˮ �� �۶���ʱ���ӹ۲�Һ�� �� |