��Ŀ����
ij�о���ѧϰС��Ϊȷ��ij������ʽ̼��þ��Ʒ����ɣ���Ƴ�����ͼ��ʾ��ʵ��װ�ã�ͼ��A��D�IJ��֣���[��֪��ʽ̼��þMgx��OH��y��CO3��z��x��y��zΪ�������������ֽܷ���������þ��ˮ�Ͷ�����̼]
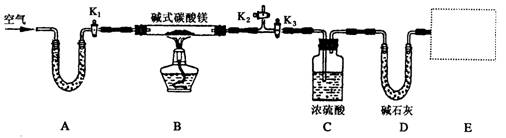
��1������ͼ���г�����δ��������װ��ʵ��װ�ú�Ӧ���Ƚ��еIJ�����__________��A��U�ι�ʢ�ŵ�ҩƷ��__________________,������Ϊ_______________________��
��2��ָ����ʦ��������Ʒ�����ָ����Ҫ��E������һװ�ã�����Ϊ��װ��Ӧ����_____________________________.
��3������ǰ��Ҫ���װ�û���������������ӣ���Ŀ����___________________����ʱ�Ի����IJ��������ǣ�����____________________���رջ���_________________��
��4���رջ���K1��K2����K3������һ��ʱ���ͬѧ�Ƿ���C��ϴ��ƿ�м���û������ð������������_________________________��
��5����Ӧ��ȫ��K1���ٻ���������������ӣ���Ŀ����_____________________��
��6��ʵ�����������£���ʽ̼��þ��Ʒ22��6 g����ӦǰCװ�õ�����Ϊ87��6 g����Ӧ������Ϊ89��4 g����ӦǰDװ�õ�����Ϊ74��7 g����Ӧ������Ϊ83��5 g��������Ƶ��ü�ʽ̼��þ�Ļ�ѧʽ______________________���ü�ʽ̼��þ���ȷֽ�Ļ�ѧ����ʽΪ____________��
����15�֣�
��1�� ���װ�õ������ԣ� ��ʯ�ң��������ƣ��� ��ȥ����װ�õĿ�������������ˮ�����Ͷ�����̼����3�֣�ÿ��1�֣�
��2�� ʢ�м�ʯ�ң��������ƣ��ĸ���ܡ���2�֣�
��3�� ����װ���к���ˮ�����Ͷ�����̼�Ŀ�������2�֣�K1��K2��1�֣� ��K3��1�֣� ��
(4) Bװ���еķ�Ӧ����ȫ����1�֣�
��5�� ����Ӧ���ɵIJ�����װ���е�ˮ�����Ͷ�����̼ȫ���ϵ�װ��C��D�С���1�֣�
��6�� Mg3(OH)2(CO3)2[�� Mg(OH)2��2MgCO3]��
Mg3(OH)2(CO3)2 ![]() 3MgO + 2CO2��+H2O ��4�֣�ÿ��2�֣�
3MgO + 2CO2��+H2O ��4�֣�ÿ��2�֣�

 �Ķ��쳵ϵ�д�
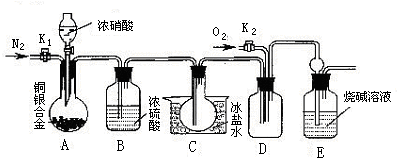
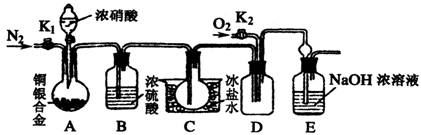
�Ķ��쳵ϵ�д�ij�о���ѧϰС���ͬѧΪ��֤Ũ������ͭ�ܷ�Ӧ��ϡ����ܣ��������ͼ��ʾװ������̽������6.4 g ͭƬ����0.2 moL���ʵ�18.4 mol/LŨ�������Բ����ƿ�й��ȣ�ֱ������������Ϊֹ�����ٶ��ڴ˹�����Һ������䣩

��1����ͬѧ��ΪҪ֤ʵ�������ۣ���Ӧ����ϡ������ͭƬ��ϼ���ʵ�飬����Ϊ���ޱ�Ҫ����˵��������________________
��2�������Լ����ܹ�������һ��֤����Ӧ���������ƿ��ȷ��������ǣ�_____
A������ ?????? B���� ???? ?? C���Ȼ�����Һ ???????? D������
��3��Ϊ�����ⶨ��������ʵ���Ũ�ȣ��ס�����λͬѧ������������ƣ�
����ͬѧ��������Aװ������һ������ͨ�����������壨�ٶ����ɵ�����ȫ���ݳ������Ȳⶨ���ɵ�SO2������Ȼ�����ʣ�������Ũ������������������ַ������ⶨSO2������
������ �����������建��ͨ��������ϡ�����ữ��KMnO4��Һ���ټ�������BaCl2��Һ�����ˡ�ϴ�ӡ��������������
������ �����建��ͨ���������ᱵ��Һ�У�Ȼ����ˡ�ϴ�ӡ��������������
����ϸ���������в�����֮��������д�±�(���Բ���������
��� | ��������ԭ�� | ��� ��ƫ�ͻ�ƫ�ߣ� | �ı��ʩ |
������ |
|
|
|
������ |
|
|
|
����ѧ����Ƶķ����ǣ��������Ӧ�����Һ��������ˮϡ����1000 mL��ȡ20.00 mL����ƿ�У�����2��3�η�ָ̪ʾ�����ñ�NaOH��Һ���еζ�(��֪������ͭ��ʼ������pHԼΪ5)�����ַ����ܷ���������Ũ�ȣ�������_______________________________________��
��4����������������е�ʵ�鷽�������ⶨ��������ʵ���Ũ�ȣ���Ҫд���������輰��Ҫ�ⶨ�����ݣ����ؼ���������д����ϸ����_____________________________________________________��