��Ŀ����
�㶫ʡ���ŷḻ�ĺ�����Դ����ˮ��ȡʳ�κ�Br2�Ժ����±���������Ʊ�������MgCl2��MgO����±�к���Mg2+��Cl-������������Na+��Fe2+��Fe3+��SO42-��CO(NH2)2�ȡ��Ʊ���������ͼ��
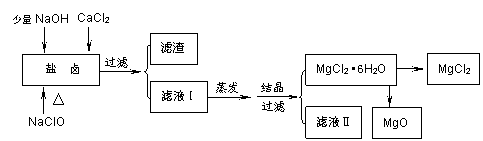
��1�������ijɷ����������������������������������������������� ��д��ѧʽ������Һ����������Ҫ�������������������������������������������� ��д���ӷ��ţ���
��2����NaClO��ȥ����CO(NH2)2ʱ������������⣬�����ܲ������ѭ�������ʣ���÷�Ӧ�Ļ�ѧ����ʽΪ���������������������������������������� ������ ������NaClO����һ���������������������������������� ���� ��
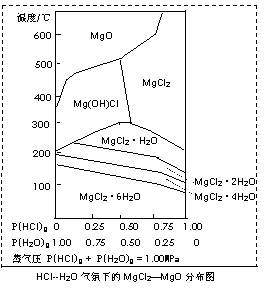
��3���¶Ⱥ�ѹǿP(HCl)g��MgCl2?6H2O�����ȷֽ�����Ӱ����ͼ��ʾ����ش��������⣺
��д��P(HCl)g = 0.25MPa���¶ȴ�300�����ߵ�550��ʱ��Ӧ�Ļ�ѧ����ʽ������������������������������������������������������������ ���� ��
��ʵ�������У���MgCl2?6H2O������ȵ�600��Ĺ����м����ò�����ˮMgCl2����ԭ���������������������������������� �� ����Ҫ�õ���ˮMgCl2���ȡ�Ĵ�ʩ������������������������������������ ���� ��
��1��Fe(OH)3 ��CaSO4 ��(2��)�� Na+������Ca2+����SO42- ��1�֣���(2��)
��2��3NaClO + CO(NH2)2 =3NaCl + CO2 ��+ N2��+ 2H2O ��
NaOH +3NaClO + CO(NH2)2 =3NaCl + NaHCO3+ N2��+ 2H2O (3��)
�� ��Fe2+ ����ΪFe3+ �����γ�Fe(OH)3 ����ȥ�� (2��)
��3����Mg��OH��Cl MgO + HCl������������������ (3��)
���� ��P��HCl��С������ʱMg2+ �ᷢ��ˮ�⡡���� (2��)��
ͨ��HCl ������P��HCl��������Mg2+ ˮ�⡣ (2��)

 ���Ͱ�ͨ��ĩ���ϵ�д�
���Ͱ�ͨ��ĩ���ϵ�д�


