��Ŀ����
ij�о���ѧϰС��̽�����л�ѧ��Ӧ��������
(I)KMnO4+KI+H2SO4��MnSO4+I2+KIO3+K2SO4+H2O
(II)FeS+HNO3��Fe(NO3)3+Fe2(SO4)3+NO2��+N2O4��+NO��+H2O
(III)CuSO4��CuO+SO3��+SO2��+O2��
(IV)CO2+KOH��KHCO3+K2CO3+H2O
��1���������ѧ��Ӧ�����ʵĻ�ѧ������֮���ǹ̶��ģ���������ѧ��Ӧ�Ļ�ѧ������֮���Dz�ȷ���ģ������ʵĻ�ѧ�������ж��顣��������ʵ��������һ�仰���ɻ�ѧ�������ж���Ļ�ѧ��Ӧ���ص㣺__________________________��
��2����Ӧ�����л�ԭ������____________________���������ʵ���֮��ȷ�����÷�Ӧ�Ļ�ѧ������Ҳ��֮ȷ����
��3��������Ϊ��Ӧ������������Ӧʽ�ӺϵĽ���������������������ѧ��Ӧ�Ӻ϶��ɵģ�
___________________________��_____________________________���ڷ�Ӧ�����У����ݵ���ת���غ㣬����__________��___________�����ʵ���֮���ǹ̶��ġ�
��4������16gCuSO4�����ĩ����Ӧ������ȫ�ֽ⣬���������������ʵ�����ȡֵ��Χ�ǣ�
________________________��
��5������Ӧ��������KHCO3��K2CO3�Ļ�ѧ�������ֱ�Ϊa��b������a��b��ʾ�÷�Ӧ�Ļ�ѧ�������Ļ�ѧ����ʽ�ǣ�__________________________��
(I)KMnO4+KI+H2SO4��MnSO4+I2+KIO3+K2SO4+H2O
(II)FeS+HNO3��Fe(NO3)3+Fe2(SO4)3+NO2��+N2O4��+NO��+H2O
(III)CuSO4��CuO+SO3��+SO2��+O2��
(IV)CO2+KOH��KHCO3+K2CO3+H2O
��1���������ѧ��Ӧ�����ʵĻ�ѧ������֮���ǹ̶��ģ���������ѧ��Ӧ�Ļ�ѧ������֮���Dz�ȷ���ģ������ʵĻ�ѧ�������ж��顣��������ʵ��������һ�仰���ɻ�ѧ�������ж���Ļ�ѧ��Ӧ���ص㣺__________________________��
��2����Ӧ�����л�ԭ������____________________���������ʵ���֮��ȷ�����÷�Ӧ�Ļ�ѧ������Ҳ��֮ȷ����
��3��������Ϊ��Ӧ������������Ӧʽ�ӺϵĽ���������������������ѧ��Ӧ�Ӻ϶��ɵģ�
___________________________��_____________________________���ڷ�Ӧ�����У����ݵ���ת���غ㣬����__________��___________�����ʵ���֮���ǹ̶��ġ�
��4������16gCuSO4�����ĩ����Ӧ������ȫ�ֽ⣬���������������ʵ�����ȡֵ��Χ�ǣ�
________________________��
��5������Ӧ��������KHCO3��K2CO3�Ļ�ѧ�������ֱ�Ϊa��b������a��b��ʾ�÷�Ӧ�Ļ�ѧ�������Ļ�ѧ����ʽ�ǣ�__________________________��
��1��һ����Ӧ����Ϊ2��������Ӧ�ļӺ�
��2��NO2��N2O4��NO
��3��CuSO4 CuO+SO3����2SO3
CuO+SO3����2SO3 2SO2+O2��SO2��O2
2SO2+O2��SO2��O2
��4����0.1��0.15��
��5��(a+b)CO2+(a+2b)KOH==aKHCO3+bK2CO3+(a/2+b)H2O
��2��NO2��N2O4��NO
��3��CuSO4
 CuO+SO3����2SO3
CuO+SO3����2SO3 2SO2+O2��SO2��O2
2SO2+O2��SO2��O2��4����0.1��0.15��
��5��(a+b)CO2+(a+2b)KOH==aKHCO3+bK2CO3+(a/2+b)H2O

��ϰ��ϵ�д�
�����Ŀ
 Fe2++Ag+����ش��������⣺
Fe2++Ag+����ش��������⣺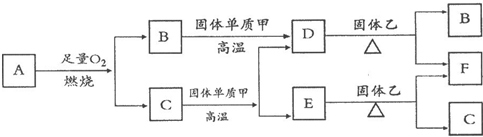

 CO2��SO2��NOx�ǶԻ���Ӱ��ϴ�����壬���ƺ�����CO2��SO2��NOx�ǽ������ЧӦ����������⻯ѧ��������Ч;����
CO2��SO2��NOx�ǶԻ���Ӱ��ϴ�����壬���ƺ�����CO2��SO2��NOx�ǽ������ЧӦ����������⻯ѧ��������Ч;���� CO+3H2
CO+3H2