��Ŀ����
I���ס���Ԫ�صĵ��ʺͻ�����Ӧ�ù㷺��
��1��������뽹̿��ʯӢɰ��ϣ��ڵ�¯�м��ȵ�1500�����ɰ��ף���ӦΪ��
2Ca3��PO4��2��6SiO2 6CaSiO3��P4O10 10C��P4O10
6CaSiO3��P4O10 10C��P4O10 P4��10CO
P4��10CO
ÿ����1mol P4ʱ������__________mol���ӷ���ת�ơ�
��2����������ƣ�Na2S2O3���dz��õĻ�ԭ������ά����C����ѧʽC6H8O6����ˮ��Һ�м������I2��Һ��ʹά����C��ȫ������ʣ���I2��Na2S2O3��Һ�ζ����ɲⶨ��Һ��ά����C�ĺ����������ķ�ӦΪ��C6H8O6��I2 C6H6O6��2H����2I�� 2
C6H6O6��2H����2I�� 2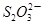 ��I2
��I2
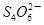 ��2I����һ�������ijά����C��Һ�м���a mol��L��1 I2��ҺV1mL����ַ�Ӧ����Na2S2O3��Һ�ζ�ʣ���I2, ����b mol��L��1Na2S2O3��ҺV2mL������Һ��ά����C�����ʵ�����___________mol��
��2I����һ�������ijά����C��Һ�м���a mol��L��1 I2��ҺV1mL����ַ�Ӧ����Na2S2O3��Һ�ζ�ʣ���I2, ����b mol��L��1Na2S2O3��ҺV2mL������Һ��ά����C�����ʵ�����___________mol��
��3����������Һ�У�����أ�KIO3�����������ƿɷ������·�Ӧ��2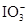 ��5
��5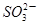 ��2H��
��2H�� I2��5
I2��5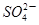 ��H2O���ɵĵ�����õ�����Һ���飬���ݷ�Ӧ��Һ������ɫ�����ʱ���������÷�Ӧ�����ʡ�ijͬѧ���ʵ�����±���ʾ��
��H2O���ɵĵ�����õ�����Һ���飬���ݷ�Ӧ��Һ������ɫ�����ʱ���������÷�Ӧ�����ʡ�ijͬѧ���ʵ�����±���ʾ��
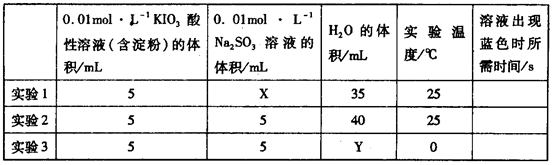
��ʵ���Ŀ����______________������X��__________mL
��ϡ��Ԫ���DZ����ս����Դ���ҹ����̲�����������λ��
��4���棨Ce���ǵؿ��к�����ߵ�ϡ��Ԫ�ء��ڼ���������CeCl3����ˮ�⣬��ˮCeCl3���ü���CeCl3��6H2O��NH4Cl��������ķ������Ʊ�������NH4Cl��������_______________________.
��5����ijǿ���Ի��ϡ����Һ�м���H2O2������pH��3��Ce3��ͨ�����з�Ӧ�γ�Ce��OH��4�������Է��롣��ɷ�Ӧ�����ӷ���ʽ��
 Ce3����
Ce3���� H2O2��
H2O2�� H2O
H2O
 Ce��OH��4����
Ce��OH��4���� ______________
______________
��1��������뽹̿��ʯӢɰ��ϣ��ڵ�¯�м��ȵ�1500�����ɰ��ף���ӦΪ��
2Ca3��PO4��2��6SiO2
 6CaSiO3��P4O10 10C��P4O10
6CaSiO3��P4O10 10C��P4O10 P4��10CO
P4��10COÿ����1mol P4ʱ������__________mol���ӷ���ת�ơ�
��2����������ƣ�Na2S2O3���dz��õĻ�ԭ������ά����C����ѧʽC6H8O6����ˮ��Һ�м������I2��Һ��ʹά����C��ȫ������ʣ���I2��Na2S2O3��Һ�ζ����ɲⶨ��Һ��ά����C�ĺ����������ķ�ӦΪ��C6H8O6��I2
 C6H6O6��2H����2I�� 2
C6H6O6��2H����2I�� 2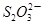 ��I2
��I2
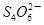 ��2I����һ�������ijά����C��Һ�м���a mol��L��1 I2��ҺV1mL����ַ�Ӧ����Na2S2O3��Һ�ζ�ʣ���I2, ����b mol��L��1Na2S2O3��ҺV2mL������Һ��ά����C�����ʵ�����___________mol��
��2I����һ�������ijά����C��Һ�м���a mol��L��1 I2��ҺV1mL����ַ�Ӧ����Na2S2O3��Һ�ζ�ʣ���I2, ����b mol��L��1Na2S2O3��ҺV2mL������Һ��ά����C�����ʵ�����___________mol����3����������Һ�У�����أ�KIO3�����������ƿɷ������·�Ӧ��2
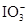 ��5
��5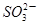 ��2H��
��2H�� I2��5
I2��5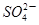 ��H2O���ɵĵ�����õ�����Һ���飬���ݷ�Ӧ��Һ������ɫ�����ʱ���������÷�Ӧ�����ʡ�ijͬѧ���ʵ�����±���ʾ��
��H2O���ɵĵ�����õ�����Һ���飬���ݷ�Ӧ��Һ������ɫ�����ʱ���������÷�Ӧ�����ʡ�ijͬѧ���ʵ�����±���ʾ�� 
��ʵ���Ŀ����______________������X��__________mL
��ϡ��Ԫ���DZ����ս����Դ���ҹ����̲�����������λ��
��4���棨Ce���ǵؿ��к�����ߵ�ϡ��Ԫ�ء��ڼ���������CeCl3����ˮ�⣬��ˮCeCl3���ü���CeCl3��6H2O��NH4Cl��������ķ������Ʊ�������NH4Cl��������_______________________.
��5����ijǿ���Ի��ϡ����Һ�м���H2O2������pH��3��Ce3��ͨ�����з�Ӧ�γ�Ce��OH��4�������Է��롣��ɷ�Ӧ�����ӷ���ʽ��
 Ce3����
Ce3���� H2O2��
H2O2�� H2O
H2O
 Ce��OH��4����
Ce��OH��4���� ______________
______________
��

��ϰ��ϵ�д�
�����Ŀ
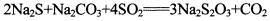 o
o

 ����KMnO4��Һ������NaHCO3��Һ��Ʒ����Һ������ʯ��ˮ
����KMnO4��Һ������NaHCO3��Һ��Ʒ����Һ������ʯ��ˮ
 ��Ϊ�ⶨ���Ƶþ���Ĵ��ȣ���С���Ե�����ָʾ������
��Ϊ�ⶨ���Ƶþ���Ĵ��ȣ���С���Ե�����ָʾ������ �ĵ�ˮ���ж��ȡ���ζ������
�ĵ�ˮ���ж��ȡ���ζ������ �ĺ���ԼΪ102%���������Լ��������������������ý������ܵ�ԭ����______________��
�ĺ���ԼΪ102%���������Լ��������������������ý������ܵ�ԭ����______________��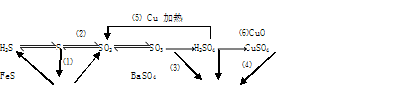
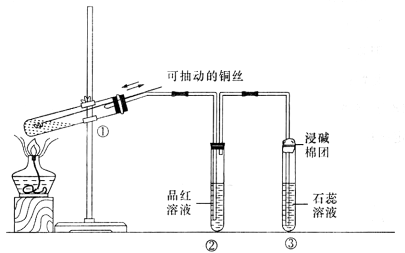
 ���Ѣ��Թ��е������ﵹ��ˮ�з�����Һ����
���Ѣ��Թ��е������ﵹ��ˮ�з�����Һ����