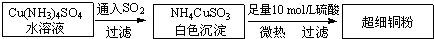��Ŀ����
CuCl��CuCl2������Ҫ�Ļ���ԭ�ϣ����������������ϡ����������������ȡ���֪��
��CuCl������CuCl2���ʵ��Ļ�ԭ����SO2��SnCl2�Ȼ�ԭ�Ƶã�
![]()
��CuCl2��Һ���Ҷ���(H2N-CH2-CH2-NH2)���γ������ӣ�
��ش��������⣺
��1����̬Cuԭ�ӵĺ�������Ų�ʽΪ ��H��N��O����Ԫ�صĵ縺���ɴ�С��˳���� ��
��2��SO2���ӵĿռ乹��Ϊ ����SnCl4��Ϊ�ȵ������һ�����ӵĻ�ѧʽΪ ��
��3���Ҷ��������е�ԭ�ӹ�����ӻ�����Ϊ ���Ҷ��������װ�[N(CH3)3]�����ڰ������Ҷ��������װ��ķе�ߵĶ࣬ԭ���� ��
��4���������γɵ��������к��еĻ�ѧ�������� ��(����ĸ)
A ��λ�� B ���Լ� C ���Ӽ� D �Ǽ��Լ�
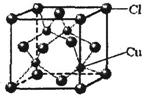
��5��CuCl�ľ����ṹ����ͼ��ʾ������Clԭ�ӵ���λ��Ϊ ��
��1��1s22s22p63s23p63d104s1��[Ar]3d10 4s1��2�֣� O >N >H��1�֣�
��2��V�Σ�1�֣� SO42-��SiO44-�ȣ�2�֣�
��3��sp3�ӻ���1�֣� �Ҷ������Ӽ�����γ���������װ����Ӽ䲻���γ������1�֣�
��4��abd��2�֣� ��5��4��2�֣�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�


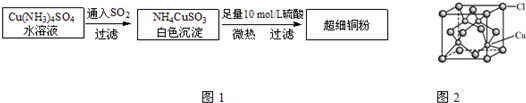
 ͭ���ʼ��仯�����ںܶ���������Ҫ����;�������ͭ����������ߵ��£���ϸͭ�ۿ�Ӧ���ڵ�����ϡ������������У�CuCl��CuCl2������Ҫ�Ļ���ԭ�ϣ����������������ϡ����������������ȣ�
ͭ���ʼ��仯�����ںܶ���������Ҫ����;�������ͭ����������ߵ��£���ϸͭ�ۿ�Ӧ���ڵ�����ϡ������������У�CuCl��CuCl2������Ҫ�Ļ���ԭ�ϣ����������������ϡ����������������ȣ�