��Ŀ����
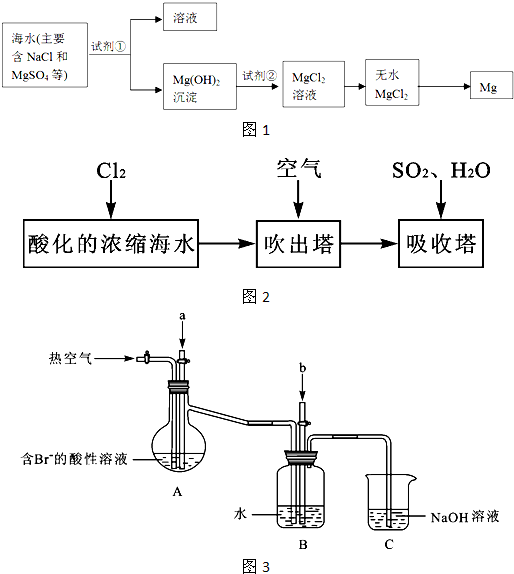
����Լռ����������71%������ʮ�־�Ŀ���DZ����ijУ�о���ѧϰС��ͬѧ�Ծ���������������Ũ����ˮ(��Ҫ��NaCl��MgSO4)�����о���
��1��ʵ��ʱ������1000mL0��20mol/LNaOH��Һ�������������NaOH��������
��3��ȡ��������������Ũ����ˮ100mL������200mL0��20mol/LNaOH��Һ��ǡ�ð����е�Mg2+��ȫ��������Ũ����ˮ��Mg2+�����ʵ���Ũ��Ϊ���١�
��3���о�С��ͬѧ������������ȫת��Ϊ��ˮMgCl2����������״̬�½��е�⣬�ɵñ�״����Cl2��������
��1��ʵ��ʱ������1000mL0��20mol/LNaOH��Һ�������������NaOH��������
��3��ȡ��������������Ũ����ˮ100mL������200mL0��20mol/LNaOH��Һ��ǡ�ð����е�Mg2+��ȫ��������Ũ����ˮ��Mg2+�����ʵ���Ũ��Ϊ���١�
��3���о�С��ͬѧ������������ȫת��Ϊ��ˮMgCl2����������״̬�½��е�⣬�ɵñ�״����Cl2��������
��1��w(NaOH) =0.20mol/L��1L��40g/mol=8g
��2��c(Mg2+) =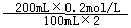 = 0.2mol/L
= 0.2mol/L
��3��V(Cl2)=0.02mol/L��0.1L��22.4L/mol=0.448L
��2��c(Mg2+) =
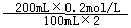 = 0.2mol/L
= 0.2mol/L ��3��V(Cl2)=0.02mol/L��0.1L��22.4L/mol=0.448L

��ϰ��ϵ�д�
 ϰ�⾫ѡϵ�д�
ϰ�⾫ѡϵ�д�
�����Ŀ
����Լռ����������71%��������п������õIJ���������ͼ��ʾ������˵��������ǣ�������
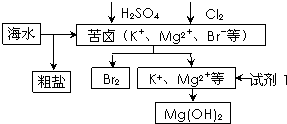

| A������BaCl2��Һ��ȥ�����е�SO42- | B���ӿ�±����ȡBr2�ķ�Ӧ�����ӷ���ʽΪ��2Br-+Cl2�T2Cl-+Br2 | C���Լ�1����ѡ��ʯ���� | D����ҵ�ϣ��������MgOұ������þ |



 ��2012?������һģ������Լռ����������71%��������п������õIJ���������ͼ��ʾ������˵������ȷ���ǣ�������
��2012?������һģ������Լռ����������71%��������п������õIJ���������ͼ��ʾ������˵������ȷ���ǣ�������