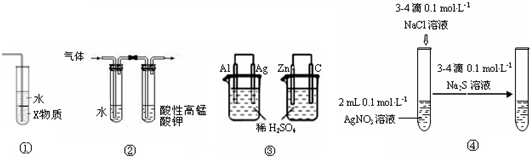
��Ŀ����
��2011?������һģ�������������壨FeSO4?7H2O����ҽҩ������Ѫ����ij����С��ⶨ�ò�Ѫ������Ԫ�صĺ�����ʵ�鲽�����£�
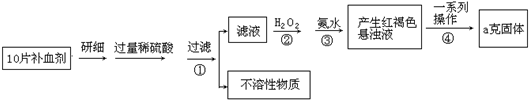
��ش��������⣺
��1��֤���������Һ�к���Fe2+�ķ�����
��2��д�����е����ӷ�Ӧ����ʽ��
��3��������з�Ӧ�����ӷ���ʽ��
��4���������һϵ�д����IJ������裺���ˡ�
��5����С����Щͬѧ��Ϊ��KMnO4��Һ�ζ�Ҳ�ܽ�����Ԫ�غ����IJⶨ����5Fe2++MnO4-+8H+��5Fe3++Mn2++4H2O��
��ʵ��ǰ������Ҫ��ȷ����һ�����ʵ���Ũ�ȵ�KMnO4��Һ250mL������ʱ��Ҫ��������ҩ�ס���ƽ�������룩���������ձ�����ͷ�ι��⣬����
�ڵζ����յ�ʱ����ɫΪ
��6��������ÿ��Ӧ����16.8mg���ҵ��������о���������ʳ����ȫ��ͨ�����ú�FeSO4?7H2O��Ƭ����������������������ÿ������ú�

��ش��������⣺
��1��֤���������Һ�к���Fe2+�ķ�����
ȡ������Һ�����Թ��У��ȵμ�KSCN��Һ����Һ����ɫ���ٵμ���ˮ����˫��ˮ��ϡ���ᣩ����Һ��ΪѪ��ɫ
ȡ������Һ�����Թ��У��ȵμ�KSCN��Һ����Һ����ɫ���ٵμ���ˮ����˫��ˮ��ϡ���ᣩ����Һ��ΪѪ��ɫ
����2��д�����е����ӷ�Ӧ����ʽ��
2Fe2++H2O2+2H+�T2Fe3++2H2O
2Fe2++H2O2+2H+�T2Fe3++2H2O
����3��������з�Ӧ�����ӷ���ʽ��
Fe3++3NH3?H2O=Fe��OH��3��+3NH4+
Fe3++3NH3?H2O=Fe��OH��3��+3NH4+
����4���������һϵ�д����IJ������裺���ˡ�
ϴ��
ϴ��
�����ա���ȴ����������5����С����Щͬѧ��Ϊ��KMnO4��Һ�ζ�Ҳ�ܽ�����Ԫ�غ����IJⶨ����5Fe2++MnO4-+8H+��5Fe3++Mn2++4H2O��
��ʵ��ǰ������Ҫ��ȷ����һ�����ʵ���Ũ�ȵ�KMnO4��Һ250mL������ʱ��Ҫ��������ҩ�ס���ƽ�������룩���������ձ�����ͷ�ι��⣬����
250mL����ƿ
250mL����ƿ
���ڵζ����յ�ʱ����ɫΪ
��
��
ɫ����6��������ÿ��Ӧ����16.8mg���ҵ��������о���������ʳ����ȫ��ͨ�����ú�FeSO4?7H2O��Ƭ����������������������ÿ������ú�
83.4
83.4
mgFeSO4?7H2OƬ����������������ͼ��֪����ʵ��ԭ��Ϊ����ҩƷ�е�Fe2+�γ���Һ����Fe2+����ΪFe3+��ʹFe3+ת��Ϊ����������������ת��Ϊ��������ͨ���ⶨ�����������������㲹Ѫ������Ԫ�صĺ�����
��1��Fe3+��KSCN��Һ�Ժ�ɫ�����������ڼ���Fe3+���ڣ����Լ�����������Fe2+����ΪFe3+������Fe2+�����ȵμ�KSCN��Һ����Һ����ɫ���ٵμ���ˮ��˫��ˮ����Һ��ΪѪ��ɫ��˵������Fe2+��
��2��˫��ˮ���������ԣ������������ܽ�Fe2+ȫ������ΪFe3+��ͬʱ����ˮ��
��3��������ǽ�Fe3+ת��Ϊ��������������
��4���������һϵ�д�������������������Һ����ת��Ϊ����������Ҫ���ˡ�ϴ�ӵ�����������Ȼ��������������������ȴ�������������������
��5���پ�ȷ����һ�����ʵ���Ũ�ȵ�KMnO4��Һ250mL������ʱ��Ҫ����������ƽ���������ձ�����ͷ�ιܣ�250mL����ƿ��
�ڸ������Ϊ��ɫ�����ζ����յ�ʱ��Fe2+����ȫ��������������һ�θ�����ز���Ӧ����Һ����ɫΪ��ɫ��
��6��������Ԫ���غ��֪16.8mg����ΪFeSO4?7H2OƬ�����������������ݻ�ѧʽ����Ԫ�����������ļ���������FeSO4?7H2O��Ƭ����������
��1��Fe3+��KSCN��Һ�Ժ�ɫ�����������ڼ���Fe3+���ڣ����Լ�����������Fe2+����ΪFe3+������Fe2+�����ȵμ�KSCN��Һ����Һ����ɫ���ٵμ���ˮ��˫��ˮ����Һ��ΪѪ��ɫ��˵������Fe2+��
��2��˫��ˮ���������ԣ������������ܽ�Fe2+ȫ������ΪFe3+��ͬʱ����ˮ��
��3��������ǽ�Fe3+ת��Ϊ��������������
��4���������һϵ�д�������������������Һ����ת��Ϊ����������Ҫ���ˡ�ϴ�ӵ�����������Ȼ��������������������ȴ�������������������
��5���پ�ȷ����һ�����ʵ���Ũ�ȵ�KMnO4��Һ250mL������ʱ��Ҫ����������ƽ���������ձ�����ͷ�ιܣ�250mL����ƿ��
�ڸ������Ϊ��ɫ�����ζ����յ�ʱ��Fe2+����ȫ��������������һ�θ�����ز���Ӧ����Һ����ɫΪ��ɫ��
��6��������Ԫ���غ��֪16.8mg����ΪFeSO4?7H2OƬ�����������������ݻ�ѧʽ����Ԫ�����������ļ���������FeSO4?7H2O��Ƭ����������
����⣺��1��Fe3+��KSCN��Һ�Ժ�ɫ�����������ڼ���Fe3+���ڣ����Լ�����������Fe2+����ΪFe3+������Fe2+�����ȵμ�KSCN��Һ����Һ����ɫ���ٵμ���ˮ��˫��ˮ����Һ��ΪѪ��ɫ��˵������Fe2+��
�ʴ�Ϊ��ȡ������Һ�����Թ��У��ȵμ�KSCN��Һ����Һ����ɫ���ٵμ���ˮ����˫��ˮ��ϡ���ᣩ����Һ��ΪѪ��ɫ��
��2��˫��ˮ���������ԣ������������ܽ�Fe2+ȫ������ΪFe3+��ͬʱ����ˮ����Ӧ���ӷ���ʽΪ2Fe2++H2O2+2H+�T2Fe3++2H2O��
�ʴ�Ϊ��2Fe2++H2O2+2H+�T2Fe3++2H2O��
��3��������ǽ�Fe3+ת��Ϊ����������������Ӧ���ӷ���ʽΪFe3++3NH3?H2O=Fe��OH��3��+3NH4+��
�ʴ�Ϊ��Fe3++3NH3?H2O=Fe��OH��3��+3NH4+��
��4���������һϵ�д�������������������Һ����ת��Ϊ����������Ҫ���ˡ�ϴ�ӵ�����������Ȼ��������������������ȴ�������������������
�ʴ�Ϊ��ϴ�ӣ���ȴ��
��5���پ�ȷ����һ�����ʵ���Ũ�ȵ�KMnO4��Һ250mL������ʱ��Ҫ����������ƽ���������ձ�����ͷ�ιܣ�250mL����ƿ��
�ʴ�Ϊ��250mL����ƿ��
�ڸ������Ϊ��ɫ�����ζ����յ�ʱ��Fe2+����ȫ��������������һ�θ�����ز���Ӧ����Һ����ɫΪ��ɫ���ʴ�Ϊ���ϣ�
��6��16.8mg����ΪFeSO4?7H2OƬ��������������������ҪFeSO4?7H2OƬ������Ϊ16.8mg��
=83.4mg��
�ʴ�Ϊ��83.4mg��
�ʴ�Ϊ��ȡ������Һ�����Թ��У��ȵμ�KSCN��Һ����Һ����ɫ���ٵμ���ˮ����˫��ˮ��ϡ���ᣩ����Һ��ΪѪ��ɫ��
��2��˫��ˮ���������ԣ������������ܽ�Fe2+ȫ������ΪFe3+��ͬʱ����ˮ����Ӧ���ӷ���ʽΪ2Fe2++H2O2+2H+�T2Fe3++2H2O��
�ʴ�Ϊ��2Fe2++H2O2+2H+�T2Fe3++2H2O��
��3��������ǽ�Fe3+ת��Ϊ����������������Ӧ���ӷ���ʽΪFe3++3NH3?H2O=Fe��OH��3��+3NH4+��
�ʴ�Ϊ��Fe3++3NH3?H2O=Fe��OH��3��+3NH4+��
��4���������һϵ�д�������������������Һ����ת��Ϊ����������Ҫ���ˡ�ϴ�ӵ�����������Ȼ��������������������ȴ�������������������
�ʴ�Ϊ��ϴ�ӣ���ȴ��
��5���پ�ȷ����һ�����ʵ���Ũ�ȵ�KMnO4��Һ250mL������ʱ��Ҫ����������ƽ���������ձ�����ͷ�ιܣ�250mL����ƿ��
�ʴ�Ϊ��250mL����ƿ��
�ڸ������Ϊ��ɫ�����ζ����յ�ʱ��Fe2+����ȫ��������������һ�θ�����ز���Ӧ����Һ����ɫΪ��ɫ���ʴ�Ϊ���ϣ�
��6��16.8mg����ΪFeSO4?7H2OƬ��������������������ҪFeSO4?7H2OƬ������Ϊ16.8mg��
| 56 |
| 278 |
�ʴ�Ϊ��83.4mg��
���������⿼��ѧ����ʵ��ԭ����ʵ����������⡢���ʷ����ᴿ��Ԫ�ػ��������ʡ�������ԭ�ζ�Ӧ�á���ѧ����ȣ��Ѷ��еȣ����ʵ��ԭ���ǽ���Ĺؼ�����Ҫѧ���߱���ʵ�Ļ���֪ʶ���ۺ�����֪ʶ�������⡢��������������

��ϰ��ϵ�д�
�����Ŀ
��2011?������һģ���������������Ʊ��Ȼ�ͭ���Ƚ�Ũ����������������80�����ң��������뺬��FeO���ʵ�CuO�ۣ���ַ�Ӧ��ʹ���ܽ⣮��֪��FeS������ˮ����������ڳ�ȥ��Һ�е�Fe2+ʱ���ɲ��õķ����ǣ�������
|
��2011?������һģ������ʵ���ͼ���Ľ��ۻ������ȷ���ǣ�������
|