��Ŀ����
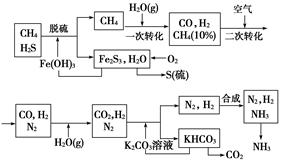
������Ȼ���ϳɰ��Ĺ�������ʾ��ͼ���£�
�����������̣����������գ�
��1��ͼ��CH4�ĵ�һ��ת�������еĻ�ѧ����ʽ��
��2����������У�����n mol Fe2O3?H2Oת����������S�����ʵ���Ϊ
��3����������������ѭ����һ��K2CO3��aq��ѭ��������N2��H2ѭ����������ѭ���б�ѭ��������
��4�����ù���NaOH��Һ������Ȼ���е����⣬��ʯī���缫������պ�������Һ�ɻ����������ܷ�Ӧ����ʽ������������������ԭ��Ϊ

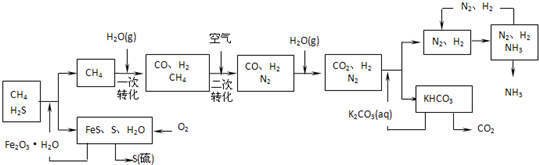
�����������̣����������գ�
��1��ͼ��CH4�ĵ�һ��ת�������еĻ�ѧ����ʽ��
CH4+H2O?CO+3H2
CH4+H2O?CO+3H2
����2����������У�����n mol Fe2O3?H2Oת����������S�����ʵ���Ϊ
n
n
mol���ú�n�Ĵ���ʽ��ʾ������3����������������ѭ����һ��K2CO3��aq��ѭ��������N2��H2ѭ����������ѭ���б�ѭ��������
Fe2O3?H2O
Fe2O3?H2O
����4�����ù���NaOH��Һ������Ȼ���е����⣬��ʯī���缫������պ�������Һ�ɻ����������ܷ�Ӧ����ʽ������������������ԭ��Ϊ
Na2S+2H2O
2NaOH+S+H2��
| ||
Na2S+2H2O
2NaOH+S+H2��
���÷������ŵ���
| ||
�÷���NaOH����ѭ�����ã�ͬʱ��ø���Ʒ����
�÷���NaOH����ѭ�����ã�ͬʱ��ø���Ʒ����
����������1�����ݹ�������ʾ��ͼ��֪CH4�ĵ�һ��ת��������������CO��H2��
��2������H2S����Fe2O3?H2O��Ӧ��3H2S+Fe2O3?H2O=2FeS+S+4H2O�����㣻
��3��������������ͼ�����漰�����ʵ���Դ��ȥ��ش�
��4������NaOH��Һ������Ȼ���е���������Na2S�����ʱ���������ӷŵ����ɵ��������������ӷŵ������������÷���NaOH����ѭ�����ã�ͬʱ��ø���Ʒ������
��2������H2S����Fe2O3?H2O��Ӧ��3H2S+Fe2O3?H2O=2FeS+S+4H2O�����㣻
��3��������������ͼ�����漰�����ʵ���Դ��ȥ��ش�
��4������NaOH��Һ������Ȼ���е���������Na2S�����ʱ���������ӷŵ����ɵ��������������ӷŵ������������÷���NaOH����ѭ�����ã�ͬʱ��ø���Ʒ������
����⣺��1��CH4�ĵ�һ��ת��������������CO��H2������ʽΪ��CH4+H2O?CO+3H2��
��2����H2S����Fe2O3?H2O��Ӧ��3H2S+Fe2O3?H2O=2FeS+S+4H2O��֪����nmolFe2O3?H2Oת��������S�����ʵ���Ϊnmol��
�ʴ�Ϊ��n��
��3���ɹ�������ʾ��ͼ��֪������ѭ���б�ѭ��������Fe2O3?H2Oѭ����
�ʴ�Ϊ��Fe2O3?H2O��
��4��NaOH��Һ������Ȼ���е���������Na2S�����ʱ���������ӷŵ����ɵ��������������ӷŵ���������������ܷ�Ӧ����ʽΪ��Na2S+2H2O
2NaOH+S+H2�����÷���NaOH����ѭ�����ã�ͬʱ��ø���Ʒ������
�ʴ�Ϊ��Na2S+2H2O
2NaOH+S+H2�����÷���NaOH����ѭ�����ã�ͬʱ��ø���Ʒ������
��2����H2S����Fe2O3?H2O��Ӧ��3H2S+Fe2O3?H2O=2FeS+S+4H2O��֪����nmolFe2O3?H2Oת��������S�����ʵ���Ϊnmol��
�ʴ�Ϊ��n��
��3���ɹ�������ʾ��ͼ��֪������ѭ���б�ѭ��������Fe2O3?H2Oѭ����
�ʴ�Ϊ��Fe2O3?H2O��
��4��NaOH��Һ������Ȼ���е���������Na2S�����ʱ���������ӷŵ����ɵ��������������ӷŵ���������������ܷ�Ӧ����ʽΪ��Na2S+2H2O
| ||
�ʴ�Ϊ��Na2S+2H2O
| ||
������������һ����ѧ��ҵ�������ϵ���Ŀ���漰��������ԭ��Ӧ������ʽ����д�����ԭ��������ѧ�������ͽ��������������Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ