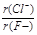��Ŀ����
(12��)���ֶ�����Ԫ�ص�ԭ�Ӱ뾶��ijЩ���ϼۼ��±���
�����ϱ����й����ݣ��������ѧ����֪ʶ���ش��������⡣�漰����Ԫ�صĴ𰸣�����Ԫ�ط��ű�ʾ��
(1)EԪ�������ڱ���λ�� ���� �壻
(2)A��H��J��Ӧ�����Ӱ뾶�ɴ�С��˳���ǣ���д���ӷ��ţ�
(3)J������B������ȼ�տ����ɻ�����X��X�ĵ���ʽ�� �����������Ļ�ѧ������Ϊ ��
(4)I���ʿ���D�������������ȼ�գ��漰�Ļ�ѧ����ʽΪ�� ��
(5)B��H���γɵĻ�������J������������Ӧˮ����Y����Һ������Ӧ�����ӷ���ʽΪ ��
| Ԫ�ش��� | A | B | D | E | G | H | I | J |
| ���ϼ� | �C1 | �C2 | +4���C4 | +4���C2 | +5���C3 | +3 | +2 | +1 |
| ԭ�Ӱ뾶��nm | 0.071 | 0.074 | 0.077 | 0.102 | 0.110 | 0.143 | 0.160 | 0.186 |
(1)EԪ�������ڱ���λ�� ���� �壻
(2)A��H��J��Ӧ�����Ӱ뾶�ɴ�С��˳���ǣ���д���ӷ��ţ�
(3)J������B������ȼ�տ����ɻ�����X��X�ĵ���ʽ�� �����������Ļ�ѧ������Ϊ ��
(4)I���ʿ���D�������������ȼ�գ��漰�Ļ�ѧ����ʽΪ�� ��
(5)B��H���γɵĻ�������J������������Ӧˮ����Y����Һ������Ӧ�����ӷ���ʽΪ ��
(1) �� ��A����1�֣� (2) F��> Na�� > Al3����2�֣�
(3)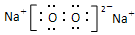 ��2�֣� ���Ӽ����Ǽ��Լ���2�֣�
��2�֣� ���Ӽ����Ǽ��Լ���2�֣�
(4) 2Mg + CO2 2MgO + C��2�֣� (5) Al2O3 + 2OH��= 2AlO2��+ H2O��2�֣�
2MgO + C��2�֣� (5) Al2O3 + 2OH��= 2AlO2��+ H2O��2�֣�
(3)
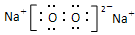 ��2�֣� ���Ӽ����Ǽ��Լ���2�֣�
��2�֣� ���Ӽ����Ǽ��Լ���2�֣�(4) 2Mg + CO2
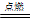 2MgO + C��2�֣� (5) Al2O3 + 2OH��= 2AlO2��+ H2O��2�֣�
2MgO + C��2�֣� (5) Al2O3 + 2OH��= 2AlO2��+ H2O��2�֣�����Ԫ�������ɵ�Ӧ�á�����Ԫ�ص���Ҫ���ϼۺ�ԭ�Ӱ뾶��֪��A��J�ֱ���F��O��C��S��P��Al��Mg��Na��
��1����Ԫ��λ�ڵ������ڵڢ�A��
��2��A��H��J��Ӧ�����ӵĺ�������Ų���ͬ�������Ӱ뾶��ԭ���������������С���������Ӱ뾶��С˳����F��> Na�� > Al3����
��3������������ȼ���������ǹ������ƣ��������ӻ�����������Ӽ��ͷǼ��Լ���
��4��þ������CO2��ȼ�գ���������þ��̼����ӦʽΪ2Mg + CO2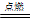 2MgO + C��
2MgO + C��
��5����������������������������������κ�ˮ��Ҳ������ǿ�������κ�ˮ����ӦʽΪAl2O3 + 2OH��= 2AlO2��+ H2O��
��1����Ԫ��λ�ڵ������ڵڢ�A��
��2��A��H��J��Ӧ�����ӵĺ�������Ų���ͬ�������Ӱ뾶��ԭ���������������С���������Ӱ뾶��С˳����F��> Na�� > Al3����
��3������������ȼ���������ǹ������ƣ��������ӻ�����������Ӽ��ͷǼ��Լ���
��4��þ������CO2��ȼ�գ���������þ��̼����ӦʽΪ2Mg + CO2
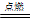 2MgO + C��
2MgO + C����5����������������������������������κ�ˮ��Ҳ������ǿ�������κ�ˮ����ӦʽΪAl2O3 + 2OH��= 2AlO2��+ H2O��

��ϰ��ϵ�д�
�����Ŀ



 ������
������