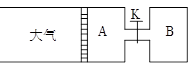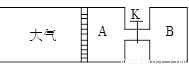��Ŀ����
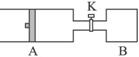
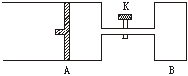
��8�֣�����ͼ��ʾ����A�г���1mol X��1mol Y����B�г���2mol X��2mol Y����ʼʱA��B�������Ϊa L������ͬ�¶Ⱥ��д��������£��������и��Է�����Ӧ��
X(g)+Y(g) ?2Z(g)+W(g)������ӦΪ���ȷ�Ӧ���ﵽƽ��ʱA���������Ϊ1.2a L��
(1) A��X��ת���ʦ�A=
(2) A��B��X��ת���ʦ�A ��B���� >��< �� = ��
(3) ��K��һ��ʱ���ִﵽƽ��ʱA�����Ϊ L����ͨ��������������Բ��ƣ�
(4) ��(3)�ﵽƽ���ͬʱ�ȷ�����A��B���¶ȣ��ﵽ��ƽ���A����� ������С�䣩
��1��40%��2��> ��3��2.6a L ��4����С
����

��ϰ��ϵ�д�
�����Ŀ