��Ŀ����
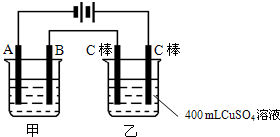
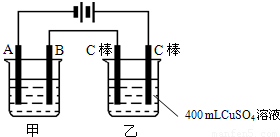
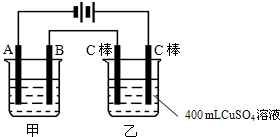 ��ͼΪΪ�����ļס��������أ��Իش�
��ͼΪΪ�����ļס��������أ��Իش���1�����׳����õ��ԭ�������϶�������A��
��
��
����B�ĵ缫��������
��
���缫��ӦʽΪAg-e-=Ag+
Ag-e-=Ag+
��Ӧѡ�õĵ������Һ��AgNO3��Һ
AgNO3��Һ
����2����������������4.32g�����ҵ����������Ϸų��������ڱ�״���µ������
448
448
mL����3�����ҳ���ʣ����Һ��Ϊ400mL��������������ʵ���Ũ��Ϊ
0.1
0.1
mol?L-1����Һ��pHΪ13
13
�����������ݵ�Դ�����������ж�A��B��Fe��C�ֱ�Ϊ���ص��������������������������������Ϸ�����ԭ��Ӧ���������Ϸ���������Ӧ���ҳط�Ӧ��������2H2O+2e-�TH2��+20H-��������2Cl--2e-�TCl2��������Ϊ�õ��ԭ������Ƭ������װ�ã�������ӦΪ��Ag++e-=Ag��������ӦΪ��Ag-e-=Ag+���������ҺӦѡ��Ʋ������ͬ�������ӵĿ���������Һ��
���ݵ缫��Ӧ�Լ�������������ת�Ƶ�����Ŀ��ȼ��㣮
���ݵ缫��Ӧ�Լ�������������ת�Ƶ�����Ŀ��ȼ��㣮
����⣺��1�����ݵ�Դ�����������ж�A��B��Fe��C�ֱ�Ϊ���ص��������������������������������Ϸ�����ԭ��Ӧ���������Ϸ���������Ӧ������Ƭ�϶���ʱ���Ʋ������Ϊ���ص������������ϵĵ缫��ӦʽΪAg-e-=Ag+���Ƽ�������Ϊ���ص������������ϵĵ缫��ӦʽAg++e-=Ag���������Һ���жƲ�������ӣ�ӦΪ��������������Һ��
�ʴ�Ϊ����������Ag-e-=Ag+��AgNO3��Һ��
��2���ײ�������ӦΪAg++e-=Ag����������4.32g��ӦΪ����������n=
=0.04 mol��
ת�Ƶĵ���Ϊ0.04mol���������ش�����ת�Ƶĵ�����Ŀ��ȣ��Ҳ�������ӦΪ2Cl--2e-�TCl2����ת�Ƶĵ���Ϊ0.04molʱ����������������������ʵ���Ϊ0.02mol���ų������ڱ�״���µ����Ϊ0.02mol��22.4L/mol=0.448L=448mL��
�ʴ�Ϊ��448��
��3���ҳصĵ�ط�ӦʽΪ2NaCl+2H2O=Cl2��+H2��+2NaOH�����������ɵ�����Ϊ�������ƣ����������Ƶ����ʵ���Ϊx����
2NaCl+2H2O=Cl2��+2NaOH+H2�� ת�Ƶ���
2mol 2mol
x 0.04mol
����x=0.04mol
�������Ƶ����ʵ���Ũ��C=
=0.1mol/L��C��H+ ��=
=
mol/L=1��10-13mol/L
�ʴ�Ϊ��0.1mol?L-1��13��
�ʴ�Ϊ����������Ag-e-=Ag+��AgNO3��Һ��
��2���ײ�������ӦΪAg++e-=Ag����������4.32g��ӦΪ����������n=
| 4.32g |
| 108g/mol |
ת�Ƶĵ���Ϊ0.04mol���������ش�����ת�Ƶĵ�����Ŀ��ȣ��Ҳ�������ӦΪ2Cl--2e-�TCl2����ת�Ƶĵ���Ϊ0.04molʱ����������������������ʵ���Ϊ0.02mol���ų������ڱ�״���µ����Ϊ0.02mol��22.4L/mol=0.448L=448mL��
�ʴ�Ϊ��448��
��3���ҳصĵ�ط�ӦʽΪ2NaCl+2H2O=Cl2��+H2��+2NaOH�����������ɵ�����Ϊ�������ƣ����������Ƶ����ʵ���Ϊx����
2NaCl+2H2O=Cl2��+2NaOH+H2�� ת�Ƶ���
2mol 2mol
x 0.04mol
����x=0.04mol
�������Ƶ����ʵ���Ũ��C=
| 0.04mol |
| 0.4L |
| Kw |
| C(OH-) |
| 1��10-14 |
| 0.1 |
�ʴ�Ϊ��0.1mol?L-1��13��
���������⿼���˵��ԭ����������ԭ��Ӧ���йؼ��㣬�ѶȲ���ץס�������ص�ʧ����������ǽ���Ĺؼ���

��ϰ��ϵ�д�
 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�
�����Ŀ
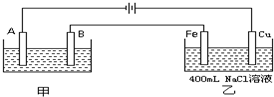
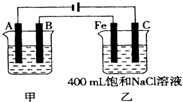 ��ͼΪ������ļס��������أ��Իش�
��ͼΪ������ļס��������أ��Իش�