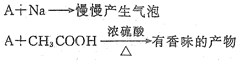��Ŀ����
���ڴ��������˵���д������
| A������ʹ��������ͬ�Ĺ�����(��OH)�����������ͬ�Ļ�ѧ���� |
| B���ǻ��������������ϵ�̼ԭ�������Ļ������Ϊ�� |
| C���Ҷ����ͱ�����������ɫ����������ζ��Һ�壬��������ˮ���Ҵ������б��������������ƻ�ױƷ |
| D����Է�����������Ĵ���������ȣ����ķе�ԶԶ���������ģ��������ڴ������봼����֮����������Ե�� |
A
A��������ʹ����������ͬ�Ĺ�����(��OH)�������������ӵIJ�λ��ͬ����OHֱ���뱽�������ķӣ������ܱ�����Ӱ�죬���ǻ��е�H�����ã������ĵ���������ӣ�����OH������������Ϊ������ʱ�ǻ���������Ӱ�죬�ǻ��ϵ�H�Ļ�Ը��������ܵ���������ӣ�

��ϰ��ϵ�д�
 ���ɿ��õ�Ԫ����AB��ϵ�д�
���ɿ��õ�Ԫ����AB��ϵ�д� С�����ϵ�д�
С�����ϵ�д�
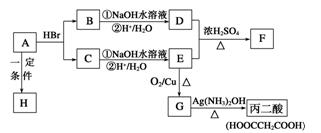
�����Ŀ
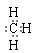
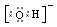
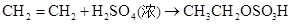 ����Ȼ����������ٽ�һ��������ϩ��
����Ȼ����������ٽ�һ��������ϩ��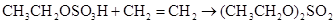 �����ټ���ˮ�����ͣ�����ˮ��Ӧ�����Ҵ���
�����ټ���ˮ�����ͣ�����ˮ��Ӧ�����Ҵ���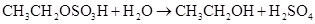
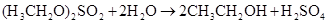
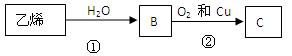
 �÷�Ӧ��������
�÷�Ӧ�������� 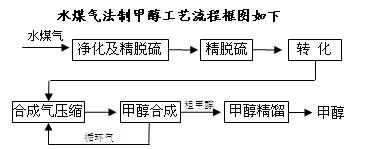
 CO��g��+H2��g�����˷�Ӧ��
CO��g��+H2��g�����˷�Ӧ�� CO (g)+2H2O (g) ��H=��519KJ/mol����ҵ��Ҫѡ����ʵĴ������ֱ��X��Y��Z���ִ�����������ʵ�飨����������ͬ��
CO (g)+2H2O (g) ��H=��519KJ/mol����ҵ��Ҫѡ����ʵĴ������ֱ��X��Y��Z���ִ�����������ʵ�飨����������ͬ��