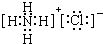��Ŀ����
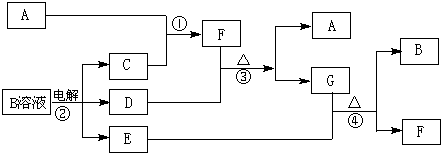
����ͼ���ʵ�ת���У�A��B��C��D��E��F�����ж�����ij�ֳ�������Ԫ�أ�����AΪ�������ʣ�B��C��D��E��FΪ������ڳ����£�X��Z��MΪ�������壬����MΪ����ɫ�����塣Y�ǻ�����ڳ�����ΪҺ�塣��ͼ���Ѱ�ת�����������еķ�Ӧ�������������
�밴Ҫ������������⣺
��1��D�ĵ���ʽ______________��
��2��д����ѧʽ��C_________��E_________
��3��д��E��Y��ⷴӦ�����ӷ���ʽ________________________��
��4��д��F��HCl��Ӧ�Ļ�ѧ����ʽ___________________________________________��
��5��д����F����Һ��ͨ��CO2�����ӷ���ʽ___________________________________��
![]()
��2��NaOH NaCl
��3��2Cl-+2H2O![]() 2OH-+Cl2��+H2��
2OH-+Cl2��+H2��
��4��Na2CO3+2HCl====2NaCl+CO2��+H2O
��5��![]() +CO2+H2O====2
+CO2+H2O====2![]()
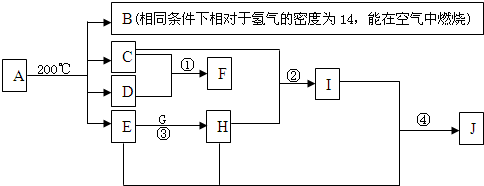
��������A��B��C��D��E��F�����ж�����ij�ֳ�������Ԫ�أ���Ŀ�������ڽ������仯�����ת���ϣ�A��������������X��Ӧ���������������ʣ�M��������A��Ӧ������������������������Ȼ�뵽A��Na��X����������Y�ǻ�����ڳ�����ΪҺ�壬���ͼ��ת����Ϣ����֪����ˮ������ʹ���ͻ�ƿڡ������ó�����ת��ͼ��


��ϰ��ϵ�д�
�����Ŀ
 ��2011?��ƽ��ģ���������糧�ͷų������ĵ������NOx������������Ͷ�����̼���������ɻ�����Ⱦ����ȼú���������������������̼�ȴ�������ʵ����ɫ���������ܼ��š��������õ�Ŀ�ģ�
��2011?��ƽ��ģ���������糧�ͷų������ĵ������NOx������������Ͷ�����̼���������ɻ�����Ⱦ����ȼú���������������������̼�ȴ�������ʵ����ɫ���������ܼ��š��������õ�Ŀ�ģ�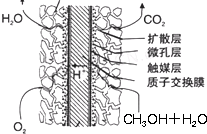
 NH3?H2O+H+
NH3?H2O+H+