��Ŀ����
A��B��C��D��EΪԭ������������������ֶ�����Ԫ�أ����н���һ�ֽ���Ԫ�ء�A��D������������ ͬ��B��C��E�����ڱ������ڣ���C��Eͬ���塣B��C������������֮�͵���D��ԭ�Ӻ����������A��C���γ����ֳ����Ļ�������ң���Է���������<�ң���
��ش��������⣺
(1)�ĵ���ʽΪ___________��д���ҵ�һ����;____________��
(2)��ij�ַ����Ľ���������̼�����缫����A��C��D ��ɵĻ�������ҺΪ�������Һ������ԭ��أ�д�������ĵ缫��Ӧʽ________________��
(3)��A��B��C����Ԫ����ɵ�ij����Һ�еμ�AgNO3��Һ���ɰ�ɫ�������÷�Ӧ�Ļ�ѧ����ʽΪ________________����Ӧ�����Һ��Ũ����������Ϊ__________��д���ӷ��ţ���
(4)д��C��D�γɵĻ�������EC2����������ԭ��Ӧ�Ļ�ѧ����ʽ�����������ת�Ƶķ������Ŀ
___________________��
��ش��������⣺
(1)�ĵ���ʽΪ___________��д���ҵ�һ����;____________��
(2)��ij�ַ����Ľ���������̼�����缫����A��C��D ��ɵĻ�������ҺΪ�������Һ������ԭ��أ�д�������ĵ缫��Ӧʽ________________��
(3)��A��B��C����Ԫ����ɵ�ij����Һ�еμ�AgNO3��Һ���ɰ�ɫ�������÷�Ӧ�Ļ�ѧ����ʽΪ________________����Ӧ�����Һ��Ũ����������Ϊ__________��д���ӷ��ţ���
(4)д��C��D�γɵĻ�������EC2����������ԭ��Ӧ�Ļ�ѧ����ʽ�����������ת�Ƶķ������Ŀ
___________________��
(1)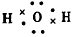 ��ɱ��������
��ɱ��������
(2)Al+4OH--3e-=AlO2-+2H2O
(3)NH4NO2+AgNO3=AgNO2��+NH4NO3��NO3-
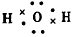 ��ɱ��������
��ɱ��������(2)Al+4OH--3e-=AlO2-+2H2O
(3)NH4NO2+AgNO3=AgNO2��+NH4NO3��NO3-
(4)


��ϰ��ϵ�д�
�����Ŀ
����ѧ--ѡ��3�����ʽṹ�����ʡ�
��������Ԫ�أ�����A��B��C��D��EΪ����������Ԫ�أ�F��GΪ��������Ԫ�أ����ǵ�ԭ����������������������������Ϣ���ش����⣮
��1��Gλ�� �� �����۵����Ų�ʽΪ ��
��2��B��̬ԭ����������ߵĵ��ӣ���������ڿռ��� ������ԭ�ӹ����
�Σ�
��3������Cԭ�ӵĵ����Ų�ͼ ��
��4����֪BA5Ϊ���ӻ����д�������ʽ ��
��5��DE3����ԭ�ӵ��ӻ���ʽΪ ���ü۲���ӶԻ��������Ʋ���ռ乹��Ϊ ��
��6���õ���ʽ��ʾFԪ����EԪ���γɻ�������γɹ��� ��
��������Ԫ�أ�����A��B��C��D��EΪ����������Ԫ�أ�F��GΪ��������Ԫ�أ����ǵ�ԭ����������������������������Ϣ���ش����⣮
| AԪ�صĺ���������͵��Ӳ�����ȣ�Ҳ����������ḻ��Ԫ�� |
| BԪ��ԭ�ӵĺ���p��������s��������1 |
| Cԭ�ӵĵ�һ�����ĵ����ֱܷ��ǣ� I1=738kJ/mol I2=1451kJ/mol I3=7733kJ/mol I4=10540kJ/mol |
| Dԭ�Ӻ�������p���ȫ������� |
| EԪ�ص������������������IJ�Ϊ4 |
| F��ǰ�������е縺����С��Ԫ�� |
| G�����ڱ��ĵ����� |
��2��B��̬ԭ����������ߵĵ��ӣ���������ڿռ���
�Σ�
��3������Cԭ�ӵĵ����Ų�ͼ
��4����֪BA5Ϊ���ӻ����д�������ʽ
��5��DE3����ԭ�ӵ��ӻ���ʽΪ
��6���õ���ʽ��ʾFԪ����EԪ���γɻ�������γɹ���
 A��B��C��D��EΪ��ѧ��ѧ�������ʣ�����A��CΪ�������ʣ�EΪ�ǽ������ʣ���ͼ������֮����ת����ϵ����ش�
A��B��C��D��EΪ��ѧ��ѧ�������ʣ�����A��CΪ�������ʣ�EΪ�ǽ������ʣ���ͼ������֮����ת����ϵ����ش�

