��Ŀ����
(1)Ϊ����Ч��չ��������Դ�����ȱ����Ƶ����۵����������пɹ������ֽϾ�������Դ�ɳ������õ��������ķ�����
| A�����ˮ | B��п��ϡ���ᷴӦ |
| C����⺣ˮ | D����ʯ�͡���Ȼ��Ϊԭ�� |
C(g)��O2(g)===CO2(g) ��H=��393.5 kJ��mol��1
H2(g)��
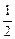 O2(g)===H2O(l) ��H=��285.8 kJ��mol��1
O2(g)===H2O(l) ��H=��285.8 kJ��mol��1��ͨ������˵����������������̼ȼ��ʱ���������ı���___________��
(3)����Դ�п���ʵ����Դ�����棬Ҳ�п���ʵ�־��á���Ч�����͡��о��������ɽ������⻯��(�ֳƼ���⻯��)���������⻯���У���ԭ������ڽ����ľ����϶֮�䣬����ɲ��̶���ͨ���Ƿǻ�ѧ�����ģ��磺LaH2.76��TiH1.73��CeH2.69��ZrH1.98��PrH2.85��TaH0.78����֪��״���£�1������ٷ۴�Լ������896���������(�ٷ۵��ܶ�Ϊ10.64 g/cm3�����ԭ������Ϊ106.4)����д����(Pd)���⻯��Ļ�ѧʽ___________��
(1)C (2)4.36��1 (3)PdH0.8
(1)���ˮ�����������������ģ��������������ȡ�������ǽϾ�������Դ�ɳ������õ����ⷽ����
(2)���Ȼ�ѧ����ʽ��֪����ͬ������������̼��ȫȼ��ʱ�ų�������֮�ȣ�
(285.8 kJ��mol��1�� )��(393.5 kJ��mol��1��
)��(393.5 kJ��mol��1��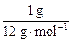 )=4.36��1
)=4.36��1
(3)�������֪��1 cm3�ٷۿ�����896 cm3��������
Pd��H=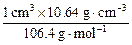 ��
�� ��2=1��0.8
��2=1��0.8
���⻯��Ļ�ѧʽΪPdH0.8
(2)���Ȼ�ѧ����ʽ��֪����ͬ������������̼��ȫȼ��ʱ�ų�������֮�ȣ�
(285.8 kJ��mol��1��
 )��(393.5 kJ��mol��1��
)��(393.5 kJ��mol��1��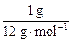 )=4.36��1
)=4.36��1(3)�������֪��1 cm3�ٷۿ�����896 cm3��������
Pd��H=
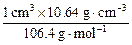 ��
�� ��2=1��0.8
��2=1��0.8���⻯��Ļ�ѧʽΪPdH0.8

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
 NH4Cl(s)
NH4Cl(s) C(s) =
C(s) =  O2(g)��H2O(l) ��H 3����285.8kJ/mol
O2(g)��H2O(l) ��H 3����285.8kJ/mol