��Ŀ����
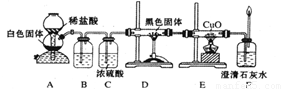
(16��)ij��ѧС���������������������װ�ã���ͼ�����Ի������Ʊ�����ϩ��
��֪��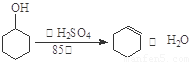
|
|
�ܶ� ��g/cm3�� |
�۵� ���棩 |
�е� ���棩 |
�ܽ��� |
|
������ |
0.96 |
25 |
161 |
������ˮ |
|
����ϩ |
0.81 |
��103 |
83 |
������ˮ |
��1���Ʊ���Ʒ
��12.5mL�����������Թ�A�У��ټ���1mLŨ���ᣬҡ�Ⱥ�������Ƭ��������������Ӧ��ȫ�����Թ�C�ڵõ�����ϩ��Ʒ��
��A�����Ƭ�������� ������B���˵�������е������� ��
���Թ�C���ڱ�ˮԡ�е�Ŀ���� ��
��2���Ʊ���Ʒ
�ٻ���ϩ��Ʒ�к��л������������������ʵȡ����뱥��ʳ��ˮ�������á��ֲ㣬����ϩ�� �㣨��ϡ����¡�������Һ���� �������ţ�ϴ�ӡ�
A��KMnO4��Һ B��ϡH2SO4 C��Na2CO3��Һ
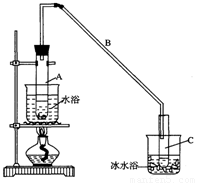
���ٽ�����ϩ��ͼװ��������ȴˮ�� �ڽ��롣����ʱҪ������ʯ�ң�Ŀ����:���� ���� ��
���ռ���Ʒʱ�����Ƶ��¶�Ӧ�� ���ң�ʵ���ƵõĻ���ϩ��Ʒ�����������۲��������ܵ�ԭ���� ��
A������ʱ��70�濪ʼ�ռ���Ʒ B��������ʵ���������� C���Ʊ���Ʒʱ���������Ʒһ������
��3���������ֻ���ϩ��Ʒ�ʹ�Ʒ�ķ������������� ��
A�������Ը��������Һ B���ý����� C���ⶨ�е�
(16��) (1)�� ��ֹ����(2��)�� ����(2��)�� �ڷ�ֹ����ϩ�Ļӷ���(2��)
(2) ����(1��)�� C��(1��)�� �ڡ�g (1��)�� ��ȥˮ�֡�(2��)
�� 83��(2��)�� �� C��(1��)��3��B��C��(2�֣�©ѡ��1�֣��д�ѡ���÷�)
��������

 ����Ӣ��ϵ�д�
����Ӣ��ϵ�д� ��
�� �п���ȼ�ա�
�п���ȼ�ա�
 ��C����������ˮ������������_
��C����������ˮ������������_
 l2����Ư���ԡ�
l2����Ư���ԡ�
 ��
�� ��ɫ����������ɵó��Ľ�����
��ɫ����������ɵó��Ľ�����  ��
��