��Ŀ����
��18�֣���ҵ�ϴӵ�⾫��ͭ�������ࣨ��������ͭ�����ȵ��ʣ�����ȡ����ʪ�������������£�
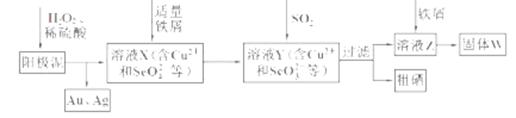
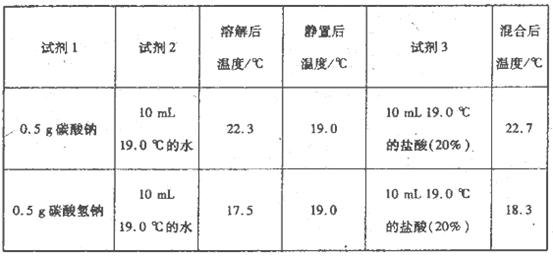
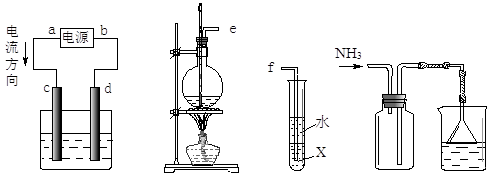
��1������ҺX�м�����м��������______ ���˲����в��ܼ���������۵�ԭ����______��
��2��������ҺZ�������ӵIJ���������______��
��3�����˲�����Ҫ�õ��������������������õ���������ʵ��������______��
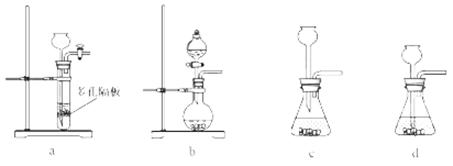
��4��ʵ��������ȡSO2��ԭ��Ϊ��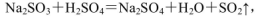 ���˴�Ӧʹ��______�����Ũ�����ᡱ��ϡ���ᡱ����ԭ���� ______����ȡSO2��װ�ã����ѡ����ͼ�е�______��
���˴�Ӧʹ��______�����Ũ�����ᡱ��ϡ���ᡱ����ԭ���� ______����ȡSO2��װ�ã����ѡ����ͼ�е�______��
��5�����������ĺ����������·����ⶨ��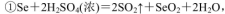
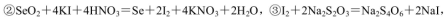 ͨ����Na2S2O3����Һ���Լ��ԣ��ζ���Ӧ�������ɵ�I2���������ĺ������ζ��������õ��IJ���������_______��ʵ����ȷ����0.1200g������Ʒ���ζ�������0.2000mol
ͨ����Na2S2O3����Һ���Լ��ԣ��ζ���Ӧ�������ɵ�I2���������ĺ������ζ��������õ��IJ���������_______��ʵ����ȷ����0.1200g������Ʒ���ζ�������0.2000mol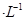 ��Na2S2O3��Һ27.60mL���������Ʒ��������������Ϊ ��
��Na2S2O3��Һ27.60mL���������Ʒ��������������Ϊ ��

��1������ҺX�м�����м��������______ ���˲����в��ܼ���������۵�ԭ����______��
��2��������ҺZ�������ӵIJ���������______��
��3�����˲�����Ҫ�õ��������������������õ���������ʵ��������______��
��4��ʵ��������ȡSO2��ԭ��Ϊ��
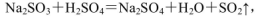 ���˴�Ӧʹ��______�����Ũ�����ᡱ��ϡ���ᡱ����ԭ���� ______����ȡSO2��װ�ã����ѡ����ͼ�е�______��
���˴�Ӧʹ��______�����Ũ�����ᡱ��ϡ���ᡱ����ԭ���� ______����ȡSO2��װ�ã����ѡ����ͼ�е�______��
��5�����������ĺ����������·����ⶨ��
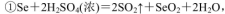
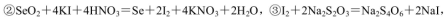 ͨ����Na2S2O3����Һ���Լ��ԣ��ζ���Ӧ�������ɵ�I2���������ĺ������ζ��������õ��IJ���������_______��ʵ����ȷ����0.1200g������Ʒ���ζ�������0.2000mol
ͨ����Na2S2O3����Һ���Լ��ԣ��ζ���Ӧ�������ɵ�I2���������ĺ������ζ��������õ��IJ���������_______��ʵ����ȷ����0.1200g������Ʒ���ζ�������0.2000mol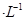 ��Na2S2O3��Һ27.60mL���������Ʒ��������������Ϊ ��
��Na2S2O3��Һ27.60mL���������Ʒ��������������Ϊ ����18�֣�
��1����SeO42-��ԭΪSeO32-��2�֣� �������ۻὫCu2+��SeO32-����ԭΪ���ʣ����������ķ��루2�֣�
��2��ȡ��ҺZ���������Թ��У��μ������ữ��BaCl2��Һ���а�ɫ�������ɣ�˵����Һ�к���SO42-��2�֣�
��3�������ᴿ��һ�����ʵ���Ũ����Һ�����ơ����ʵ��ܽ⡢��Һ��������Ũ�����ϡ�͵ȣ�2�֣�ֻҪ��ȷ���������֣�
��4����Ũ�����ᣨ2�֣� SO2������ˮ���ý�Ũ������������ SO2���ݳ���2�֣�
b��2�֣�
��5����ƿ����ʽ�ζ��ܣ�2�֣� 90.85%��2�֣�
��1����SeO42-��ԭΪSeO32-��2�֣� �������ۻὫCu2+��SeO32-����ԭΪ���ʣ����������ķ��루2�֣�
��2��ȡ��ҺZ���������Թ��У��μ������ữ��BaCl2��Һ���а�ɫ�������ɣ�˵����Һ�к���SO42-��2�֣�
��3�������ᴿ��һ�����ʵ���Ũ����Һ�����ơ����ʵ��ܽ⡢��Һ��������Ũ�����ϡ�͵ȣ�2�֣�ֻҪ��ȷ���������֣�
��4����Ũ�����ᣨ2�֣� SO2������ˮ���ý�Ũ������������ SO2���ݳ���2�֣�
b��2�֣�
��5����ƿ����ʽ�ζ��ܣ�2�֣� 90.85%��2�֣�
�����������1��H2O2Ϊǿ���������ɽ��������е�Se��������ΪSeO42-����������ͼ������������м������SeO32-��������м�������ǣ���SeO42-��ԭΪSeO32-��������������������Cu2+��SeO32-��Ӧ��������ԭΪ���ʣ����������ķ��롣
��2����ҺZ���е�������ΪSO42?�����鷽��Ϊ��ȡ��ҺZ���������Թ��У��μ������ữ��BaCl2��Һ���а�ɫ�������ɣ�˵����Һ�к���SO42-��
��3�������ᴿ����Һ���������ò��������裬ʹ��Һ�������ȣ�һ�����ʵ���Ũ����Һ�����ƣ��ò��������衢���������ʵ��ܽ⡢Ũ�����ϡ�͵ȡ�
��4����ΪSO2������ˮ���ý�Ũ������������ SO2���ݳ���������ȡSO2�ý�Ũ�����a������������ڿ�״������Ƚϴ�Ĺ�����Һ�巴Ӧ��Na2SO3Ϊ��ĩ״�����ʺϣ�b����Һ©���ʺϷ�ĩ״������Һ�巴Ӧ��Һ©���ɿ���Һ��ļ�������c������©���ĵ��ܿ���Һ�����ϣ�����ӳ���©���ݳ������ʺϣ�d������©�����ܿ���Һ��ļ���������������ʵ�Ϊb�
��5��Na2S2O3����ҺΪ���ԣ��ü��Եζ���ʢ�ţ�����Һ����ƿʢ�ţ�������Ŀ����3����ѧ����ʽ�ɵö�Ӧ��ϵ��Se ~ SeO2 ~ 2I2 ~ 4Na2S2O3���������Ʒ��������������=0.0276L��0.2000mol/L��1/4��79g/mol��0.1200g��100%=90.85%

��ϰ��ϵ�д�
 ���㼤�������100�ִ��Ծ�ϵ�д�
���㼤�������100�ִ��Ծ�ϵ�д�
�����Ŀ