��Ŀ����
X��Y��Z��W���ֻ�������ɶ�����Ԫ����ɣ�����X��������Ԫ�أ�X��Y��Z����ɫ��Ӧ��Ϊ��ɫ��WΪ��ɫ��ζ���塣�����ֻ������������ת����ϵ�����ַ�Ӧ����P��Ӧ��������ȥ����![]()
������������⣺
��1��W�ĵ���ʽ��________________________��
��2��X��Y����Һ�з�Ӧ�����ӷ���ʽ��_________________________________________��
��3��X���е�����Ԫ��֮�䣨���֡����ֻ����֣�����ɶ��ֻ����ѡ������ijЩ�����������ͼװ�ã��г̶ֹ�װ������ȥ������ʵ�飬װ�â��в�����ɫ������װ��V�п��ռ���һ����ɫ���塣

��װ�â��з�Ӧ�Ļ�ѧ����ʽ��________________________________________________��
װ�â������ʵĻ�ѧʽ��__________________________________________��
����X���е�����Ԫ���е�������ɵ�ij������ڴ����������Ʊ����ռ����������װ��V�����壬�û�����Ļ�ѧʽ��_____________��������װ����_______________________������ͼѡ���Ҫװ�ã���д��ţ���
��4����Z��Һ��ͨ�����������Ƶ�ij�������������г��õ�Ư�ס����������ʣ�ͬʱ��X���ɣ��÷�Ӧ�Ļ�ѧ����ʽ��______________________________________________________��
�������������Ĺؼ���ȷ��X������ͼʾ��ת����ϵ�������������ȷ��X��NaHCO3,Y��NaOH,Z�� Na2CO3��W��CO2�����ݣ�3��������ʵ��װ��ͼ������������ȡCO2������CO2��Na2O2��Ӧ���������ն���CO2�� ���и��V���ռ�O2��H2O2Ҳ������MnO2�������������·ֽ���O2��
�𰸣�![]()
(2)![]() +OH-====
+OH-====![]() +H2O
+H2O
��3���� Na2CO3+H2SO4====Na2SO4+CO2��+H2O��2NaHCO3+H2SO4====Na2SO4+2CO2��+2H2O Na2O2
��H2O2 ������
(4)2Na2CO3+Cl2+H2O====NaClO+NaCl+2NaHCO3

 ���ʿ��ÿ��ֳɳ�ϵ�д�
���ʿ��ÿ��ֳɳ�ϵ�д� ��2011?���գ�ԭ������С��36��X��Y��Z��W����Ԫ�أ�����X���γɻ�����������Ԫ�أ�Yԭ�ӻ�̬ʱ���������������ڲ��������2����Zԭ�ӻ�̬ʱ2pԭ�ӹ������3��δ�ɶԵĵ��ӣ�W��ԭ������Ϊ29��
��2011?���գ�ԭ������С��36��X��Y��Z��W����Ԫ�أ�����X���γɻ�����������Ԫ�أ�Yԭ�ӻ�̬ʱ���������������ڲ��������2����Zԭ�ӻ�̬ʱ2pԭ�ӹ������3��δ�ɶԵĵ��ӣ�W��ԭ������Ϊ29��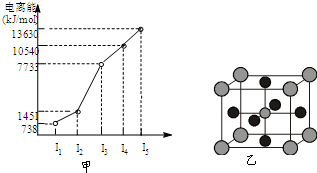 ��X��Y��Z��W����Ԫ�أ�ԭ���������μ�С����֪X�ǵ������ڵ�����Ԫ�أ��䲿�ֵ�������ͼ����ʾ��X��YԪ�ؾ�����ͬ����������ϼۣ�Z ԭ��p�������3�����ӣ�Wԭ�Ӽ۵����Ų�ʽΪnsnnpn��
��X��Y��Z��W����Ԫ�أ�ԭ���������μ�С����֪X�ǵ������ڵ�����Ԫ�أ��䲿�ֵ�������ͼ����ʾ��X��YԪ�ؾ�����ͬ����������ϼۣ�Z ԭ��p�������3�����ӣ�Wԭ�Ӽ۵����Ų�ʽΪnsnnpn��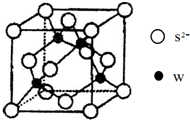 ԭ������С��36��X��Y��Z��W����Ԫ�أ�����Xԭ�ӻ�̬ʱ2pԭ�ӹ������3��δ�ɶԵ��ӣ�Yԭ�ӻ�̬ʱ���������������ڲ��������3����ZԪ�ص�����������ˮ�����������ǿ��W��ԭ������Ϊ30��
ԭ������С��36��X��Y��Z��W����Ԫ�أ�����Xԭ�ӻ�̬ʱ2pԭ�ӹ������3��δ�ɶԵ��ӣ�Yԭ�ӻ�̬ʱ���������������ڲ��������3����ZԪ�ص�����������ˮ�����������ǿ��W��ԭ������Ϊ30��