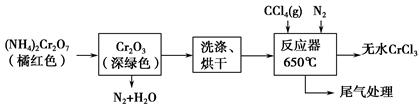
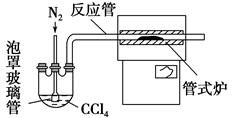
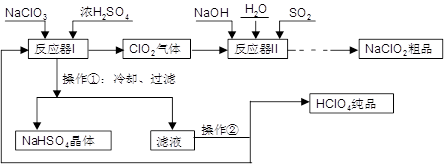
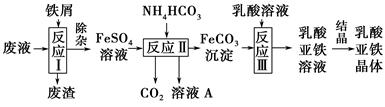
��Ŀ����
Ϊ��̽��AgNO3�����ȶ��ԣ�ij��ѧ��ȤС�����������ʵ�顣����ͼ��ʾ��ʵ��װ��A����AgNO3���壬��������ɫ���壬��װ��D���ռ�����ɫ���塣����Ӧ�������Թ��в�������Ϊ��ɫ��
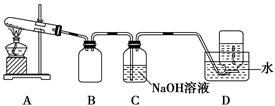
(1)װ��B��������________��
(2)��С�����۲���֤����ɫ����ΪO2������֤������________________________________________��
(3)���������ϡ�Ag2O�ͷ�ĩ״��Ag��Ϊ��ɫ��Ag2O�����ڰ�ˮ��
��������롿�Թ��в����ĺ�ɫ��������ǣ���.Ag����.Ag2O����.Ag��Ag2O��
��ʵ����֤����С��Ϊ��֤�������룬�ֱ�ȡ������ɫ��������Թ��У�����������ʵ�顣
��ʵ�����ۡ���������ʵ�飬����ȷ���������ɷֵ�ʵ����______(��ʵ����)��
��ʵ����ۡ���������ʵ��������С��ó�AgNO3�����ȷֽ�IJ�����______��

(1)װ��B��������________��
(2)��С�����۲���֤����ɫ����ΪO2������֤������________________________________________��
(3)���������ϡ�Ag2O�ͷ�ĩ״��Ag��Ϊ��ɫ��Ag2O�����ڰ�ˮ��
��������롿�Թ��в����ĺ�ɫ��������ǣ���.Ag����.Ag2O����.Ag��Ag2O��
��ʵ����֤����С��Ϊ��֤�������룬�ֱ�ȡ������ɫ��������Թ��У�����������ʵ�顣
| ʵ���� | ���� | ���� |
| a | ����������ˮ���� | ��ɫ���岻�ܽ� |
| b | ��������ϡ���ᣬ�� | ��ɫ�����ܽ⣬����������� |
��ʵ�����ۡ���������ʵ�飬����ȷ���������ɷֵ�ʵ����______(��ʵ����)��
��ʵ����ۡ���������ʵ��������С��ó�AgNO3�����ȷֽ�IJ�����______��
��(1)��������(2)�ô����ǵ�ľ�����뼯��ƿ�ڣ�ľ����ȼ��֤����ɫ����ΪO2��(3)b��Ag��NO2��O2
��(1)װ��B�еĽ����ܺͳ����ܵij�����ȣ���Ϊ��ȫƿ����ֹC�е���Һ������Aװ���У������Թ�ը�ѡ�(2)����O2�ķ������������ǵ�ľ�����뼯��ƿ�ڣ���ľ����ȼ��֤������Ϊ������(3)�������ڰ�ˮ��ʵ��a�У����백ˮ����岻�ܽ⣬˵����ɫ����ΪAg����������ϡ���ᷴӦ����NO����ʵ��b����˵��������Ag����Ag��Ag2O�Ļ�����������������ֻ֪�ܲ���ʵ��a�Ľ��ۣ���AgNO3����ֽ�IJ���ΪAg��NO2��O2��

��ϰ��ϵ�д�
 ����ѵ�����⿼ϵ�д�
����ѵ�����⿼ϵ�д�
�����Ŀ