��Ŀ����
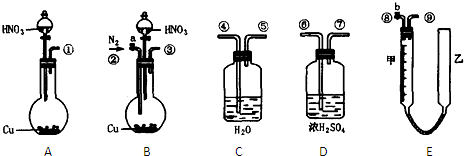
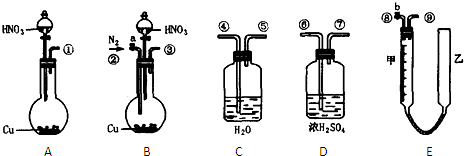
�����ͼ��ѡ�ñ�Ҫ��װ�ý��е�ⱥ��ʳ��ˮ��ʵ�飬Ҫ��ⶨ�����������������������������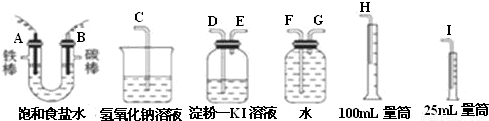
��1��A�������ĵ缫��Ӧʽ��______B�������ĵ缫��Ӧʽ��______
��2�������������ʵ��װ��ʱ�����ӿڵ���ȷ����˳��Ϊ��A��______��______��______��B��______��______��______��
��3��֤����������Cl2��ʵ��������______��
��4����֪�����ò�����H2�����Ϊ44.8mL���Ѿ�����ɱ�״������������Һ�����Ϊ50mL����ʱ��Һ��NaOH�����ʵ���Ũ��Ϊ______��
��5����һ�͵�ⱥ��ʳ��ˮ����ת���෴��װ���У�һ��ͨ����飬��һ��ͨ���������������ҺΪKOH��Һ�������ķ�ӦʽΪ______�����ķ�ӦʽΪ______��
���𰸡���������1�������У��������������ӵõ��ӣ�������������ʧ���ӵĹ��̣�
��2������ʵ���Ŀ�ĺ�װ�õ�����������װ�ã�
��3�����������û����ʵ⣬�ⵥ�������۱���ɫ��
��4�����ݹ�ʽc=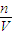 ������NaOH�����ʵ���Ũ�ȣ�
������NaOH�����ʵ���Ũ�ȣ�
��5����ȼ�ϵ���У�������ʧ���ӵ���ȼ�ϣ������ϵõ��ӵ���������
����⣺��1����ⱥ��ʳ��ˮ�������ʱ��A���ǻ��ý����缫��ӦΪ�������������������ӵõ��ӣ�
��ӦʽΪ��2H++2e-=H2����B����������������������ʧ���ӵĹ��̣���Ӧʽ�ǣ�2Cl--2e-=Cl2����
�ʴ�Ϊ��2H++2e-=H2����2Cl--2e-=Cl2����
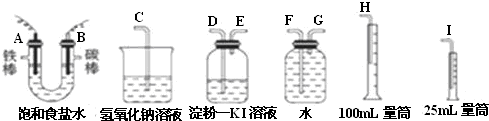
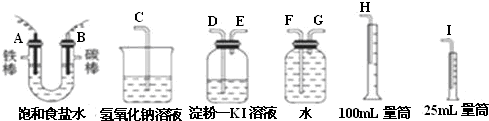
��2���ⶨ�������������������ˮ�������������Ƕ̽�����������A��G����װ�е��۵⻯����Һ��ϴ��ƿ��������ʱ������Ҫ�����̳�������B��D������Ҫ����β����������E��C���ʴ�Ϊ��G��F��H��D��E��C��
��3���������Խ��⻯���еĵ��û����������ɵĵⵥ�ʿ�ʹʹʪ��ĵ��۵⻯����ֽ����������֤����������Cl2��ʵ��������ʪ��ĵ��۵⻯����ֽ�������ʴ�Ϊ��ʪ��ĵ��۵⻯����ֽ������
��4�����ݵ�ⱥ��ʳ��ˮ�ĵ��ԭ������ʽ��2NaCl+2H2O 2NaOH+H2��+Cl2������������H2�����Ϊ44.8mL0.002molʱ�������������Ƶ����ʵ���Ϊ0.004mol��������Һ��NaOH�����ʵ���Ũ��=
2NaOH+H2��+Cl2������������H2�����Ϊ44.8mL0.002molʱ�������������Ƶ����ʵ���Ϊ0.004mol��������Һ��NaOH�����ʵ���Ũ��=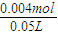 =0.08mol/L��
=0.08mol/L��
�ʴ�Ϊ��0.08mol/L��
��5���ڼ���ȼ�ϵ���У�������ʧ���ӵ���ȼ�ϼ��飬�ڼ��Ի����µ缫��ӦΪ��CH4-8e-+10OH-=CO32-+7H2O�������ϵõ��ӵ����������ڼ��Ի����µ缫��ӦΪ��2O2+8e-+4 H2O=8OH-��
�ʴ�Ϊ��CH4-8e-+10OH-=CO32-+7H2O��2O2+8e-+4 H2O=8OH-��
������������һ���ۺ�֪ʶ��Ŀ������Ƕȹ㣬Ҫ��ѧ�����з����ͽ��������������ѶȽϴ�
��2������ʵ���Ŀ�ĺ�װ�õ�����������װ�ã�
��3�����������û����ʵ⣬�ⵥ�������۱���ɫ��
��4�����ݹ�ʽc=
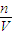 ������NaOH�����ʵ���Ũ�ȣ�
������NaOH�����ʵ���Ũ�ȣ���5����ȼ�ϵ���У�������ʧ���ӵ���ȼ�ϣ������ϵõ��ӵ���������
����⣺��1����ⱥ��ʳ��ˮ�������ʱ��A���ǻ��ý����缫��ӦΪ�������������������ӵõ��ӣ�
��ӦʽΪ��2H++2e-=H2����B����������������������ʧ���ӵĹ��̣���Ӧʽ�ǣ�2Cl--2e-=Cl2����
�ʴ�Ϊ��2H++2e-=H2����2Cl--2e-=Cl2����
��2���ⶨ�������������������ˮ�������������Ƕ̽�����������A��G����װ�е��۵⻯����Һ��ϴ��ƿ��������ʱ������Ҫ�����̳�������B��D������Ҫ����β����������E��C���ʴ�Ϊ��G��F��H��D��E��C��
��3���������Խ��⻯���еĵ��û����������ɵĵⵥ�ʿ�ʹʹʪ��ĵ��۵⻯����ֽ����������֤����������Cl2��ʵ��������ʪ��ĵ��۵⻯����ֽ�������ʴ�Ϊ��ʪ��ĵ��۵⻯����ֽ������
��4�����ݵ�ⱥ��ʳ��ˮ�ĵ��ԭ������ʽ��2NaCl+2H2O
 2NaOH+H2��+Cl2������������H2�����Ϊ44.8mL0.002molʱ�������������Ƶ����ʵ���Ϊ0.004mol��������Һ��NaOH�����ʵ���Ũ��=
2NaOH+H2��+Cl2������������H2�����Ϊ44.8mL0.002molʱ�������������Ƶ����ʵ���Ϊ0.004mol��������Һ��NaOH�����ʵ���Ũ��=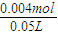 =0.08mol/L��
=0.08mol/L���ʴ�Ϊ��0.08mol/L��
��5���ڼ���ȼ�ϵ���У�������ʧ���ӵ���ȼ�ϼ��飬�ڼ��Ի����µ缫��ӦΪ��CH4-8e-+10OH-=CO32-+7H2O�������ϵõ��ӵ����������ڼ��Ի����µ缫��ӦΪ��2O2+8e-+4 H2O=8OH-��
�ʴ�Ϊ��CH4-8e-+10OH-=CO32-+7H2O��2O2+8e-+4 H2O=8OH-��
������������һ���ۺ�֪ʶ��Ŀ������Ƕȹ㣬Ҫ��ѧ�����з����ͽ��������������ѶȽϴ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ