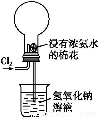
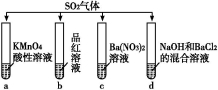
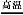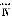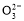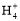��Ŀ����
�������������ѧ�γ���������ǿ��,������������ͭ�ķ�Ӧ����ش���������:
(1)��100 mL 18 mol��L-1��Ũ�����м��������ͭƬ,����ʹ֮��ַ�Ӧ,�����������ڱ�״���µ������������������(��д����);
A.7.32 LB.6.72 LC.20.16 LD.30.24 L
(2)��ʹ������Ӧ��ʣ���ͭƬ�����ܽ�,�ɽ���Һϡ�Ͳ������м���������,д����Ӧ�����ӷ���ʽ: ����
(1)AB
(2)3Cu+2N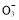 +8H+
+8H+ 3Cu2++2NO��+4H2O
3Cu2++2NO��+4H2O
��������(1)n(H2SO4)=0.1 L��18 mol��L-1=1.8 mol,���ݷ�Ӧ:2H2SO4(Ũ)+Cu CuSO4+SO2��+2H2O,������ȫ��Ӧ,����0.9 mol��H2SO4����ԭΪSO2����,���Ϊ20.16 L,��ʵ�����淴Ӧ�Ľ���,����Ũ�ȱ�С,������ͭ��Ӧ,�����ɵ�SO2���С��20.16 L,������AB��
CuSO4+SO2��+2H2O,������ȫ��Ӧ,����0.9 mol��H2SO4����ԭΪSO2����,���Ϊ20.16 L,��ʵ�����淴Ӧ�Ľ���,����Ũ�ȱ�С,������ͭ��Ӧ,�����ɵ�SO2���С��20.16 L,������AB��
(2)����NaNO3��,ʣ���ϡ������N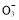 �൱�ڴ���ϡ����,����ϡ������Cu�ķ�Ӧ:
�൱�ڴ���ϡ����,����ϡ������Cu�ķ�Ӧ:
3Cu+2N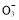 +8H+
+8H+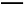 3Cu2++2NO��+4H2O��
3Cu2++2NO��+4H2O��

ijʵ��С����0.50 mol��L-1NaOH��Һ��0.50 mol��L-1������Һ�����к��ȵIJⶨ��
��.����0.50 mol��L-1NaOH��Һ
(1)��ʵ���д�ԼҪʹ��470 mL NaOH��Һ,������Ҫ����NaOH������������g��
(2)��ͼ��ѡ�����NaOH��������Ҫ��������(����ĸ):����������
���� | ������ƽ (������) | С�ձ� | ����ǯ | ������ | ҩ�� | ��Ͳ |
���� |
|
|
|
|
|
|
��� | a | b | c | d | e | f |
��.�ⶨ�к���
(1)ʵ�����ϱ����ձ�(��С�����ձ�)����ĭ���ϡ���ĭ���ϰ塢��ͷ�ιܡ����ᡢNaOH��Һ,��ȱ�ٵ�ʵ�鲣����Ʒ������������
(2)ȡ50 mL NaOH��Һ��30 mL������Һ����ʵ��,ʵ���������±���
������д�±��еĿհ�:
ʵ�� ���� | ��ʼ�¶�t1/�� | ��ֹ�¶� t2/�� | �¶Ȳ�ƽ��ֵ (t2-t1)/�� | |||
H2SO4 | NaOH | ƽ��ֵ | ||||
1 | 26.2 | 26.0 | 26.1 | 30.1 |
|
|
2 | 27.0 | 27.4 | 27.2 | 33.3 |
| |
3 | 25.9 | 25.9 | 25.9 | 29.8 |
| |
4 | 26.4 | 26.2 | 26.3 | 30.4 |
| |
�ڽ�����Ϊ0.50 mol��L-1NaOH��Һ��0.50 mol/L������Һ���ܶȶ���1 g/cm3,�кͺ�������Һ�ı�����c=��4.18��J/(g����)�����к��Ȧ�H=������������(ȡС�����һλ)��
������ʵ����ֵ�����57.3 kJ��mol-1��ƫ��,����ƫ���ԭ�������(����ĸ)����������
A.ʵ��װ�ñ��¡�����Ч����
B.��ȡNaOH��Һ�����ʱ���Ӷ���
C.�ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
D.���¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�