��Ŀ����
(14��)ijͬѧ����ˮ�ʼ��վ����960mL 1 mol��L��1NaOH��Һ�Ա�ʹ�á�
(1)��ͬѧӦѡ��________mL������ƿ��
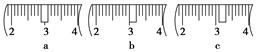
(2)�������������ͼ��ʾ������ͼ����Ӧ����ͼ�е�________(��ѡ����ĸ)֮�䡣


A������ۡ�������B������ڡ�������C�������
(3)��ͬѧӦ��ȡNaOH����________g��������Ϊ23.1 g���ձ�����������ƽ�ϳ�ȡ����NaOH����ʱ�����ڸ�����ѡȡ����������С________(����ĸ)��������ͼ��ѡ������ȷ��ʾ����λ�õ�ѡ��________(����ĸ)��
������������
(4)���в�����������Һ��Ũ�ȴ�С�к�Ӱ�죿
��ת������Һ��δϴ�Ӳ��������ձ���Ũ�Ȼ�__________(�ƫ��ƫС������Ӱ�족����ͬ)��
������ƿ��ԭ������������ˮ��Ũ�Ȼ�____________��
(1)��ͬѧӦѡ��________mL������ƿ��
(2)�������������ͼ��ʾ������ͼ����Ӧ����ͼ�е�________(��ѡ����ĸ)֮�䡣


A������ۡ�������B������ڡ�������C�������
(3)��ͬѧӦ��ȡNaOH����________g��������Ϊ23.1 g���ձ�����������ƽ�ϳ�ȡ����NaOH����ʱ�����ڸ�����ѡȡ����������С________(����ĸ)��������ͼ��ѡ������ȷ��ʾ����λ�õ�ѡ��________(����ĸ)��
������������
| | a | b | c | d | e |
| �����С/g | 100 | 50 | 20 | 10 | 5 |

(4)���в�����������Һ��Ũ�ȴ�С�к�Ӱ�죿
��ת������Һ��δϴ�Ӳ��������ձ���Ũ�Ȼ�__________(�ƫ��ƫС������Ӱ�족����ͬ)��
������ƿ��ԭ������������ˮ��Ũ�Ȼ�____________��
(1)1000 (2)C (3)40.0 bd c (4) ��ƫС ����Ӱ��
��1����������ƿ�Ĺ��û��960ml�ģ�����Ӧ��ѡ��1000ml����ƿ���ơ�
��2���ڶ���֮ǰ����Ҫ��������ˮֱ����̶���2��3cm�������Դ�ѡC��
��3����������ƿ�Ĺ����1000ml�ģ�������Ҫ�������Ƶ�������1L��1mol/L��40g/mol��40.0g���ձ����������Ƶ�����֮����63.1g������5g���������룬��������ѡ�����bd�������λ����c��
��4���������ķ������ɸ���c��n/V�����жϡ���δϴ�Ӳ��������ձ�����n��С������Ũ��ƫС��������ƿ��ԭ������������ˮ������Ӱ��n��V������Ũ�Ȳ��䡣
��2���ڶ���֮ǰ����Ҫ��������ˮֱ����̶���2��3cm�������Դ�ѡC��
��3����������ƿ�Ĺ����1000ml�ģ�������Ҫ�������Ƶ�������1L��1mol/L��40g/mol��40.0g���ձ����������Ƶ�����֮����63.1g������5g���������룬��������ѡ�����bd�������λ����c��
��4���������ķ������ɸ���c��n/V�����жϡ���δϴ�Ӳ��������ձ�����n��С������Ũ��ƫС��������ƿ��ԭ������������ˮ������Ӱ��n��V������Ũ�Ȳ��䡣

��ϰ��ϵ�д�
�����Ŀ