��Ŀ����
��100mLNaOH��Һ�м���NH4NO3��(NH4)2SO4�Ĺ���������ȳ�ַ�Ӧ����ͼ��ʾ����Ļ����������Ͳ�����������(��״��)��ϵ��
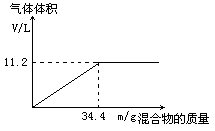
(1)�Լ���NaOH��Һ�����ʵ���Ũ�ȡ�
(2)��NaOH��Һ�����Ϊ140mL���������������Ϊ51��6g����ַ�Ӧ��������������(��״��)Ϊ������?
(3)��NaOH��Һ�����Ϊ180mL�����������������Ϊ51��6g����ַ�Ӧ��������������(��״��)Ϊ������?

(1)�Լ���NaOH��Һ�����ʵ���Ũ�ȡ�
(2)��NaOH��Һ�����Ϊ140mL���������������Ϊ51��6g����ַ�Ӧ��������������(��״��)Ϊ������?
(3)��NaOH��Һ�����Ϊ180mL�����������������Ϊ51��6g����ַ�Ӧ��������������(��״��)Ϊ������?
�⣺��1��5mol/L ��3�֣� ��2��15.68 L��3�֣���3��16.8L��3�֣�
���黯ѧ����Ĺ����벻������
��1����ͼ���֪�����Ż��������������ӣ����õ��İ�����������Ϊ11.2L
�ɷ�ӦNH4����OH�� NH3����H2O��֪��OH�������ʵ���Ϊ0.5mol��NaOH��Һ�����ʵ���Ũ��Ϊ5mol/L
NH3����H2O��֪��OH�������ʵ���Ϊ0.5mol��NaOH��Һ�����ʵ���Ũ��Ϊ5mol/L
34.4g�����������NH4������Ϊ0.5mol����NH4NO3��(NH4)2SO4�����ʵ����ֱ�Ϊx��y
80x+132y=34.4 x+2y=0.5
���x=0.1mol y=0.2mol�������ߵ����ʵ�����Ϊ1��2
��2��140mlNaOH�ṩ��OH�������ʵ���Ϊ0.7mol
�������������Ϊ51��6g���ɼ����NH4NO3��(NH4)2SO4�����ʵ����ֱ�Ϊ0.15mol��0.3mol���ṩNH4������Ϊ0.75mol
�ɷ�ӦNH4����OH�� NH3����H2O��֪��OH��������Բ��㣬���ɵİ���Ϊ0.7mol����״�������Ϊ22.4��0.7=15.68 L
NH3����H2O��֪��OH��������Բ��㣬���ɵİ���Ϊ0.7mol����״�������Ϊ22.4��0.7=15.68 L
��3��180mlNaOH�ṩ��OH�������ʵ���Ϊ0.9mol����������NH4���������㣬�����ɰ���Ϊ0.75mol����״�������Ϊ22.4��0.75=16.8L
��1����ͼ���֪�����Ż��������������ӣ����õ��İ�����������Ϊ11.2L
�ɷ�ӦNH4����OH��
 NH3����H2O��֪��OH�������ʵ���Ϊ0.5mol��NaOH��Һ�����ʵ���Ũ��Ϊ5mol/L
NH3����H2O��֪��OH�������ʵ���Ϊ0.5mol��NaOH��Һ�����ʵ���Ũ��Ϊ5mol/L34.4g�����������NH4������Ϊ0.5mol����NH4NO3��(NH4)2SO4�����ʵ����ֱ�Ϊx��y
80x+132y=34.4 x+2y=0.5
���x=0.1mol y=0.2mol�������ߵ����ʵ�����Ϊ1��2
��2��140mlNaOH�ṩ��OH�������ʵ���Ϊ0.7mol
�������������Ϊ51��6g���ɼ����NH4NO3��(NH4)2SO4�����ʵ����ֱ�Ϊ0.15mol��0.3mol���ṩNH4������Ϊ0.75mol
�ɷ�ӦNH4����OH��
 NH3����H2O��֪��OH��������Բ��㣬���ɵİ���Ϊ0.7mol����״�������Ϊ22.4��0.7=15.68 L
NH3����H2O��֪��OH��������Բ��㣬���ɵİ���Ϊ0.7mol����״�������Ϊ22.4��0.7=15.68 L��3��180mlNaOH�ṩ��OH�������ʵ���Ϊ0.9mol����������NH4���������㣬�����ɰ���Ϊ0.75mol����״�������Ϊ22.4��0.75=16.8L

��ϰ��ϵ�д�
�����Ŀ