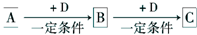��Ŀ����
��֪101kPa���У�H2(g)+1/2 O2(g)=H2O(g)����H=-241.8kJ/mol��������˵�����������ȷ���ǣ� ��
A��H2��ȼ����Ϊ241.8kJ/mol
B��H2(g)+1/2O2(g)=H2O(l)����H<-241.8kJ/mol
C��2H2(g)+O2(g)=2H2O(g)����H>-241.8kJ/mol
D��1molH2��1/2molO2����������1molH2O(g)��������
B
��������
���������ȼ��������һ�������£�1mol��ȼ����ȫȼ�������ȶ���������ʱ���ų���������ˮ���ȶ�״̬��Һ̬��A����ȷ��Һ̬ˮ������������̬ˮ����������������ȼ������Һ̬ˮʱ���ȶ࣬������Խ�࣬��HԽС��B��ȷ��ͬ������C����ȷ����Ӧ�Ƿ��ȵģ���1molH2��1/2molO2������������1molH2O(g)����������D����ȷ����ѡB��
���㣺����ȼ���ȡ���Ӧ�ȵ��й��жϺ�Ӧ��
�����������Ǹ߿��еij������ͣ������е��Ѷ�����Ŀ��顣���������ӱ��������ǿ�����ض�ѧ������֪ʶ�Ĺ��̺�ѵ����ּ�ڿ���ѧ��������û���֪ʶ���ʵ�����������������������ѧ���������������ͷ�ɢ˼ά����������Ĺؼ�����ȷȼ���Ⱥͷ�Ӧ�ȵĺ����Լ��ж����ݣ�Ȼ��������������ü��ɡ�

 �Űٷֿ�ʱ����ϵ�д�
�Űٷֿ�ʱ����ϵ�д���֪101kPa���У�H2(g)+1/2 O2(g)=H2O(g)����H=-241.8kJ/mol��������˵�����������ȷ���ǣ� ��
| A��H2��ȼ����Ϊ241.8kJ/mol |
| B��H2(g)+1/2O2(g)=H2O(l)����H<-241.8kJ/mol |
| C��2H2(g)+O2(g)=2H2O(g)����H>-241.8kJ/mol |
| D��1molH2��1/2molO2����������1molH2O(g)�������� |